Abstract
Purpose
Primary vascular dysregulation (PVD) is a condition in which the response to cold temperature or external stimuli is abnormal. We investigated whether triflusal use results in amelioration of PVD symptoms and improvement of several related parameters compared with aspirin.
Materials and Methods
Eighty-eight PVD patients (54% female, 56±8 years) were randomly selected to receive either triflusal (300 mg, b.i.d.) or aspirin (150 mg, b.i.d.) for a period of 6 weeks followed by crossover. PVD was defined as both red-blood-cell standstill in video-assisted microscopic capillaroscopy during cold stimulation using carbon dioxide gas and a score of more than 7 points in a validated questionnaire. Efficacy of treatment was assessed by 1) cold intolerance symptom severity (CISS) score, 2) finger Doppler indices, and 3) indocyanine green perfusion imaging.
Results
The use of triflusal resulted in a greater improvement in CISS score (44.5±18.4 vs. 51.9±16.2; p<0.001) and in mean radial peak systolic velocity (69.8±17.2 vs. 66.1±16.4; p=0.011) compared to aspirin. Furthermore, significant differences were also observed in perfusion rates on indocyanine green perfusion imaging between triflusal and aspirin (45.6±25.8 vs. 51.6±26.9; p=0.020).
Primary vascular dysregulation (PVD) is defined as an inherited tendency to react inappropriately to stimuli such as coldness or emotional stress, and cold extremities are the leading symptom.12 This symptom has historically been named "hiesho," cold syndrome, or vasospastic syndrome; recently, it has been termed as PVD to reflect the fact it is a functional dysregulation of the blood vessels seen among a relatively healthy population.34 The main symptoms of PVD include thermal discomfort with cold extremities, which may be accompanied by hypotension, headache, or tinnitus. The symptoms of PVD may mimic those of Raynaud's phenomenon. However, the symptoms are much milder, and the triphasic color-change characteristically seen in Raynaud's phenomenon is absent.5 Although the disease clearly causes discomfort among patients, it is not currently considered as a disease that requires a specific treatment.
Currently, the treatment of PVD involves avoiding emotional stress and cold exposure, as well as maintaining an appropriate body mass index and an intake of fruits and vegetables abundant in antioxidants. Pharmacological agents used for the treatment of PVD include magnesium, calcium channel blocker, endothelin antagonist, fludrocortisone, carbonic anhydrase inhibitor, ginkgo biloba, and others; however, there are few well-controlled studies regarding the effects of such agents on PVD, creating a need for further research.6
Current efforts elucidating the pathophysiology of PVD suggest that regulation of vascular contraction or oxidative stress may be a viable approach toward therapy. Ophthalmologic diseases associated with PVD have shown increases in endothelin-1 and resulting increases in prostaglandin E2 (PGE2), implying that pharmacologic agents targeting these molecules may ameliorate the symptoms.6
Triflusal (2-acetoxy-4-trifluoromethylbenzoic acid) is chemically related to aspirin.7 However, in contrast to aspirin,8 it leaves intact the arachidonic acid pathway,9 favors the production of nitric oxide (NO),10 and increases the concentration of cyclic nucleotide in endothelial cells, resulting in the expansion of peripheral blood vessels. In this study, we explored the efficacy of triflusal on PVD, comparing it with aspirin in a double-blind randomized crossover trial.
This study, performed from August 2011 to October 2012, enrolled patients of 20 to 60 years of age who 1) visited our outpatient clinic for symptoms of microcirculation impairment or 2) saw the advertisement listed in newspapers. PVD was defined as a combination of a score greater than 7 points in a validated questionnaire [10-question interview: subject selection symptom score (SSS score)]3 and an abnormal result of a local cold exposure test on nail fold capillaroscopy.4111213 This method has been described in detail previously.14 Briefly, the skin of the nail fold was made transparent using a drop of oil. A microscope with a monitor coupled to a video recorder was used, allowing blood flow to be saved and to be analyzed. After the baseline flow recording, the nail fold area was cooled to 14-15℃ for 60 seconds by rapidly decompressing carbon dioxide (gas stream temperature, -15℃), and the occurrence and duration of capillary blood flow was recorded. A flow stop time of greater than 12 seconds was defined as a vasospastic reaction. After a detailed history-taking of patients, we excluded individuals with a history of systemic disease (e.g., diabetes, overt peripheral artery disease, familiar hypercholesterolemia, systemic circulatory disease other than vasospasm). This study additionally excluded patients who were using vasodilators; pregnant or nursing patients; patients with bleeding tendency, thrombocytopenia (platelet<100000 mm3), chronic liver disease [aspartate transaminase (ALT)>100 IU/L or alanine transaminase (AST)>100 IU/L], or renal dysfunction (creatinine>4.0 mg/dL); and patients in whom the use or discontinuation of antiplatelet agents was contraindicated. Among the 122 initially registered patients, 12 were diagnosed as not having PVD and were excluded, leaving 110 patients who were ultimately enrolled. These patients were placed into either an aspirin group or a triflusal group after 1:1 randomization. During the study, 22 patients dropped out, while the remaining 88 patients completed the study. The reasons for dropout included gastrointestinal symptoms such as dyspepsia or stomachache (11 patients; 4 in aspirin group, 7 in triflusal group), occurrence of colon cancer (1 patient), cholecystectomy due to a gallbladder stone, foreign body sensation in the esophagus (1 patient), and withdrawal of consent (9 patients).
This study proceeded in a double-blind, randomized, crossover design. Fig. 1 describes the overall process of the study. In short, the selected patients were given either triflusal (300 mg, b.i.d.) or aspirin (150 mg, b.i.d.) for 6 weeks. After a washout period of 2 weeks, the drugs were crossed over, and the patients were given the drug they had not originally received for 6 more weeks. During each 6-week period of drug administration, the patients' cold intolerance symptom severity (CISS) scores were measured, and finger Doppler and indocyanine green perfusion imaging were performed. Institutional Review Committee approval and informed consent were obtained, and all clinical investigation was conducted according to the principles expressed in the Declaration of Helsinki. The ClinicalTrials.gov identifier was NCT01612273.
The primary endpoints were the change of CISS and the mean radial peak systolic velocity (PSV). To assess the subjective symptoms of the patients, this study employed the CISS score.15 The CISS questionnaire was originally used to measure post-traumatic cold intolerance; however, considering that it is a well-validated questionnaire that accurately reflects patients' symptoms, this study used the questionnaire to assess the subjective symptoms of PVD patients. Finger Doppler indices were measured by color Doppler ultrasound with a 7- to 15-MHz linear transducer using a Philips iE33 (Philips Medical Systems, Bothell, WA, USA). The color duplex Doppler imaging was used to visualize the flow, and the flow velocities were also measured with spectral analysis. The measured parameters were radial and palmar PSV and end diastolic velocity (EDV). PSV and EDV values were expressed as centimeters per second, and the intraclass correlation coefficient of radial finger Doppler was 88.4%.
As a secondary endpoint, indocyanine green perfusion imaging was performed using a system developed by Vieworks (Seongnam, Korea). In total, 120 images (748×518 pixels) of both hands were taken at 5-s intervals for 8 min immediately after an intravenous bolus injection of indocyanine green (0.2 mg/kg). A time-series analysis of indocyanine green fluorescence was then performed and translated into the perfusion rates of each pixel. The perfusion rate (%/min) was defined as the fraction of blood exchanged per min in the vascular volume. The perfusion rate of each pixel was represented in a perfusion map as a color-coded picture, and the distributions of the pixel perfusion rates in each hand were expressed as a histogram. Additionally, for assessment of the exploratory endpoints, Endothelin-1 levels were also measured by ELISA kits according to the manufacturer's specifications (Neogen Corp., Lexington, KY, USA).
Clinical evaluations and laboratory measurements, including serum chemistry, urinalysis, and hematology were performed at selected visits. All clinical and laboratory adverse experiences and concomitant medication use were recorded.
Safety and tolerance were evaluated by reviewing all safety variables including clinical adverse events, laboratory test results, and vital signs. Serious adverse events were immediately reported regardless of the investigator's assessment of a causal relationship to the study drugs, and all events were recorded for each subject.
No study thus far has investigated the change in microcirculation with the use of triflusal. However, according Campia, et al.,8 the use of aspirin reduces forearm blood flow by 0.5 mL/min/dL, and we hypothesized that triflusal would not result in the reduction of blood flow caused by aspirin. Based on this hypothesis, in a crossover design in which a p value of less than 0.05 was considered to be statistically significant, a two-tailed test observing a significant difference with 90% probability would require a total of 87 patients. After accounting for a 20% drop-out rate, approximately 110 patients were determined to be needed.
The baseline characteristics of the patients are summarized in Table 1. In short, the mean age of the patients was 56 years, and the proportion of females was 53%. The two groups did not show a significant difference in SSS scores during screening (8.4±1.2 vs. 8.5±1.2; p=0.675) or baseline CISS (63.7±9.9 vs. 64.4±12.0; p=0.783). The two groups also showed similar Doppler parameters, including radial PSV, radial EDV, palmar PSV, and palmar EDV. Indocyanine green perfusion scans were also not significantly different between the two groups (Table 2).
The use of triflusal significantly reduced CISS by week 6 (64.4±12.0 vs. 47.4±16.1; p<0.001), and the reduction was also observed after crossover from aspirin to triflusal (55.9±12.0 vs. 41.5±20.2; p<0.001) (Table 3). The use of aspirin also improved CISS by week 6; however, no further improvement was seen after crossover from triflusal to aspirin. Overall, the use of triflusal resulted in a significantly greater improvement in symptoms compared to aspirin (44.5±18.4 vs. 51.9±16.2; p<0.001) (Fig. 2A).
Radial PSV significantly increased from baseline after 6 weeks of triflusal administration (58.3±18.2 vs. 67.3±17.6; p<0.001). However, aspirin also showed elevated radial PSV (58.8±15.6 vs. 64.9±16.7; p=0.012), and no difference between the triflusal and aspirin groups was observed at 6 weeks (67.3±17.6 vs. 64.9±16.7; p=0.506). However, while the patients who crossed over from aspirin to triflusal showed a significant increase in radial PSV by week 14 (64.9±16.7 vs. 74.1±19.0; p<0.001), those who crossed over from triflusal to aspirin did not show an additional increase in radial PSV (67.3±17.6 vs. 67.5±19.3; p=0.936). The triflusal group did not show a greater increase in radial PSV compared to the aspirin group at week 6 or week 14; however, after combining the two results, the triflusal group did show a significant increase in radial PSV compared to the aspirin group (69.8±17.2 vs. 66.1±16.4; p=0.011) (Fig. 2B).
An increase in the indocyanine green perfusion rate compared to baseline was observed in both the triflusal and aspirin groups. However, the perfusion rates at week 6 were not significantly different between the two groups (49.4±26.6 vs. 50.2±26.5; p=0.876). After crossover, the aspirin-triflusal group show-ed a sustained increase in perfusion, while the triflusal-aspirin group perfusion rate decreased. By week 14, indocyanine green perfusion rates were significantly different between the two groups (54.0±27.4 vs. 41.1±24.5; p=0.022). Overall, the use of triflusal resulted in a clear increase in perfusion rate compared to the use of aspirin (51.6±26.9 vs. 45.6±25.8; p=0.020); however, when aspirin was used, there was no significant increase in perfusion rate compared to baseline (42.2±14.7 vs. 45.6±25.8; p=0.220) (Fig. 2C).
Plasma endothelin-1 levels also showed a trend similar to radial PSV and CISS. Both triflusal and aspirin reduced plasma endothelin-1 levels compared to baseline by week 6, although the former resulted in a greater reduction (0.77±0.28 vs. 0.99±0.40; p=0.004), and overall, plasma endothelin-1 was maintained at a lower level in the triflusal group than in the aspirin group (0.78±0.32 vs. 1.02±0.37; p<0.001) (Fig. 2D).
A total of 11 patients were withdrawn due to adverse events re-ported during this study (aspirin: 4; triflusal: 7). There were 40 cases of reported adverse events (from these, 13 were related to aspirin, and 27 were associated to triflusal) in 37 subjects during treatment. The most frequently reported adverse events were dyspepsia (12 cases related to aspirin, 24 cases related to triflusal) and esophageal irritation (1 case related to aspirin, 3 cases related to triflusal). All adverse events were of mild or moderate intensity, and all subjects recovered.
The importance of this study can be seen in three aspects. First, this study is the first to have attempted a pharmaceutical therapy to improve the symptoms of cold extremities in PVD patients. Second, this study found that triflusal is beneficial in improving cold extremities of PVD patients and that treatment results in greater improvement of microcirculation and symptoms than the use of aspirin (a representative case is shown in the Supplementary Fig. 1, only online). Lastly, this study also demonstrated a change in a biomarker-specifically, a decrease in endothelin-1, which results from decreased inflammation-along with the aforementioned beneficial effects, which may serve as an explanation of the mechanism.
PVD should be separated from other diseases such as microvascular complications of diabetes mellitus or small vessel diseases, despite the fact that they share similar pathophysiology affecting blood flow. Particularly, if a vascular dysregulation is due to diseases that can lead to increased ET-1 plasma levels, it needs to be classified as a secondary vascular dysregulation. Furthermore, small vessel diseases should not be confused with secondary vascular dysregulation.616 PVD has been reported to occur in up to 30% of the otherwise-healthy population aged 20 to 40 years. It also occurs more often in females than in males and in thin subjects more than in obese subjects,17 as well as in academics more than in blue-collar workers and in Asians more than in Caucasians. The main signs are arterial hypotension and cold extremities with an increased response to coldness.18 In addition, patients often indicate altered drug sensitivity (partly due to altered expression of ATP-binding cassette proteins),19 decreased sensations of thirst12 (ET-1 increases the PGE2 level in the center of thirst), migraines, and prolonged sleep onset time.13 These symptoms result in a decreased quality of life and problems with everyday life. In addition, there have been reports suggesting PVD as a predisposing factor of normal tension glaucoma, myocardial infarction, and cerebral infarction.4 However, PVD is not considered a disease entity as of yet; therefore, treatment for this condition is not indicated in all cases. Patients with PVD are generally in good health and do not require a specific treatment. However, treatment may be considered if the patient has PVD-related disease or if the symptoms result in difficulty performing daily activities. The side effects of the treatment agents need to be weighed against the expected benefits. Drugs such as magnesium, calcium channel blocker, and gingko biloba, which are relatively inexpensive and safe, may lead to an improved quality of life resulting from symptom relief. However, it may be difficult to recommend agents such as bosentan or fludrocortisone due to their potential side effects.6
Triflusal is an antiplatelet agent with structural similarities to salicylates, though it is not a derivative of aspirin. Triflusal has a dose-dependent inhibitory effect on platelet aggregation, and the most effective dosage for a platelet anti-aggregation effect is 600 mg/day.20 It has an efficacy similar to aspirin in patients with cerebral or myocardial infarction. In several large-scale, randomized, double-blind, multicenter studies including 2113 patients with non-disabling ischemic stroke or a transient ischemic attack in the previous 6 months, triflusal 600 mg/day was equally as effective as aspirin 325 mg/day, as evident from the incidence of the primary composite endpoint of serious vascular events (12.7% vs. 13.1%).21 The Triflusal in Myocardial Infarction (TIM) study also revealed no significant difference between triflusal and aspirin in the occurrence of the composite primary endpoint of death, nonfatal myocardial reinfarction, or nonfatal cerebrovascular events within 35 days of acute myocardial infarction (9.1% vs. 10.2%).22 Therefore, triflusal is an antiplatelet agent that can replace aspirin.
Triflusal, despite its structural analogy to aspirin, exhibits distinct pharmacological, pharmacokinetic, and biochemical properties.23 When compared to aspirin, several potential beneficial effects can be expected from triflusal. In patients with type 1 diabetes, serum levels of thromboxane B2 were greatly reduced with both triflusal and aspirin after 15 days (by 85% and 99%, respectively; p<0.05). However, reductions in serum 6-keto-prostaglandin-F 1α levels were negligible with triflusal compared with aspirin (8.8% vs. 97.8%; p<0.001).9 These data suggest that triflusal has selective activity for platelet arachidonic acid metabolism, whereas aspirin inhibits both platelet and vascular endothelial arachidonic acid metabolism. Triflusal also exerts multiple effects on platelet adhesion and aggregation.24 Although it inhibits platelet prostaglandin G/H-synthase, triflusal is a weaker inhibitor than aspirin; therefore, it does not significantly reduce prostacyclin synthesis by endothelial prostaglandin G/H-synthase.25 Both triflusal and its main active metabolite, 3-hydroxy-4-trifluoro-methylbenzoic acid, inhibit degradation of platelet and endothelial cyclic adenosine monophosphate, which subsequently limits intracellular calcium mobilization and platelet-endothelial cell interactions.2426 They also inhibit prostaglandin G/H-synthase-2 expression while activating transcription factor nuclear factor (NF)-κB and NF-κB-induced inflammatory mediators, such as vascular cell adhesion molecule-1.27 One study reported that triflusal inhibits nuclear factor of activated T-cells (NFAT)-mediated transcription at a therapeutically relevant concentration, while aspirin inhibits NFAT transactivation only at a high concentration.28 Moreover, triflusal enhances NO synthesis in neutro-phils, leading to an increased vasodilatory potential.29 A recent in vitro study found that administration of endothelin-1 in APP23 transgenic mice triggered endothelin-induced ischemia along with increases in Alzheimer's disease pathological markers in the region of the infarct, and triflusal reduced inflammation surrounding the endothelin-induced infarct.30 It appears that the vasodilation effect, anti-inflammatory effect of triflusal, and prevention of vasoconstriction caused by endothelin-1 ameliorates symptoms of PVD and improved blood circulation.
The limitations of this study are as follows. First, this study began under the assumption that aspirin results in vasoconstriction.8 However, consistent administration of aspirin actually resulted in vasodilation instead of vasoconstriction. While this is difficult to explain, a possible mechanism is that aspirin initially causes vasoconstriction yet results in vasodilation when used long-term by improving endothelial function.31 Second, this was a single center study performed mainly on Asians, which creates difficulty in generalizing the conclusion. Third, there were seasonal variations within the duration of this study, and the differences in climate may have affected the subjective reactions of PVD patients. However, this seasonal effect was shared by all patients, and we believe it did not greatly affect the objective parameters. Fourth, the incidence of dyspepsia was relatively high during triflusal use in this study. However, the rate was similar to previous reported adverse events [27.4% in the Triflusal versus Acetylsalicylic Acid in Secondary Prevention of Cerebral Infarction (TACIP) study].21 Lastly, additional studies are needed for patients who are unreactive to the drugs. Regardless of these limitations, however, it was clearly demonstrated that triflusal is superior to aspirin in increasing peripheral blood flow and in ameliorating symptoms.
This study presented data of subjective symptoms and objective parameters obtained using a double-blind, randomized, crossover design, which suggests that triflusal provides greater beneficial effects to PVD patients than aspirin. It also demonstrated a decrease in endothelin-1 during the use of triflusal to explain this beneficial phenomenon. Therefore, triflusal may be considered as a first-line treatment in patients with symptoms of PVD who are using aspirin.
Figures and Tables
 | Fig. 1Study design. This study was a double-blind, randomized, crossover design. The selected patients were given either triflusal or aspirin for 6 weeks. After a washout period of 2 weeks, the drugs were crossed over, and the patients were given the drug they had not received for 6 more weeks. CISS, cold intolerance symptom severity; PSV, peak systolic velocity; ICG, indocyanine green. |
 | Fig. 2Primary, secondary, and exploratory endpoints. (A) Although the CISS scores of Aspirin and Triflusal groups both decreased by week 6, only the CISS score of Triflusal group decreased further by week 14 after cross-over, while that of Aspirin group remained unchanged. Overall, both Aspirin and Triflusal were effective in relieving symptoms, but Triflusal group had significantly more improved symptoms compared with Aspirin group. (B) Although radial PSV was increased in both Aspirin and Triflusal groups by week 6, it was increased further only in Triflusal group by week 14 after cross-over while the Aspirin group showed no further change. Overall, although both Aspirin and Triflusal groups had increased radial PSV, the Triflusal group showed a more significantly increased PSV compared with Aspirin group. (C) ICG perfusion scan was significantly increased in the group that was treated with Triflusal compared with baseline or those treated with Aspirin. (D) ET-1 was significantly decreased in Triflusal group compared with Aspirin group by week 6, and this significant decreased was also observed after the cross-over. CISS, cold intolerance symptom severity; PSV, peak systolic velocity; ICG, indocyanine green. |
Table 1
Baseline Characteristics

Table 2
Baseline Value of Symptom Score, Doppler Parameter Indices, and Indocyanine Green Perfusion Scans
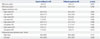
Table 3
Primary and Secondary Endpoints of Pre-Crossover, Post-Crossover, and Combined

ACKNOWLEDGEMENTS
This research was supported by the Leading Foreign Research Institute Recruitment Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT, & Future Planning (MSIP; 2012027176).
The authors thank Sae-Hwa Lee and Ran-Young Song, sonographers in the echocardiography laboratory, for their helpful assistance in collecting the complete data.
References
1. Frank SM, Raja SN, Bulcao C, Goldstein DS. Age-related thermoregulatory differences during core cooling in humans. Am J Physiol Regul Integr Comp Physiol. 2000; 279:R349–R354.

2. Pettit SE, Marchand I, Graham T. Gender differences in cardiovascular and catecholamine responses to cold-air exposure at rest. Can J Appl Physiol. 1999; 24:131–147.

3. Nagashima K, Yoda T, Yagishita T, Taniguchi A, Hosono T, Kanosue K. Thermal regulation and comfort during a mild-cold exposure in young Japanese women complaining of unusual coldness. J Appl Physiol (1985). 2002; 92:1029–1035.

4. Flammer J, Pache M, Resink T. Vasospasm, its role in the pathogenesis of diseases with particular reference to the eye. Prog Retin Eye Res. 2001; 20:319–349.

6. Flammer J, Konieczka K, Flammer AJ. The primary vascular dysregulation syndrome: implications for eye diseases. EPMA J. 2013; 4:14.

7. Murdoch D, Plosker GL. Triflusal: a review of its use in cerebral infarction and myocardial infarction, and as thromboprophylaxis in atrial fibrillation. Drugs. 2006; 66:671–692.
8. Campia U, Choucair WK, Bryant MB, Quyyumi AA, Cardillo C, Panza JA. Role of cyclooxygenase products in the regulation of vascular tone and in the endothelial vasodilator function of normal, hypertensive, and hypercholesterolemic humans. Am J Cardiol. 2002; 89:286–290.

9. De la Cruz JP, Pavia J, Garcia-Arnes J, Sanchez de la Cuesta F. Effects of triflusal and acetylsalicylic acid on platelet aggregation in whole blood of diabetic patients. Eur J Haematol. 1988; 40:232–236.

10. De Miguel LS, Jiménez A, Montón M, Farré J, Del Mar Arriero M, Rodríguez-Feo JA, et al. A 4-trifluoromethyl derivative of salicylate, triflusal, stimulates nitric oxide production by human neutrophils: role in platelet function. Eur J Clin Invest. 2000; 30:811–817.

11. Gasser P, Flammer J. Blood-cell velocity in the nailfold capillaries of patients with normal-tension and high-tension glaucoma. Am J Ophthalmol. 1991; 111:585–588.
12. Teuchner B, Orgül S, Ulmer H, Haufschild T, Flammer J. Reduced thirst in patients with a vasospastic syndrome. Acta Ophthalmol Scand. 2004; 82:738–740.
13. Pache M, Kräuchi K, Cajochen C, Wirz-Justice A, Dubler B, Flammer J, et al. Cold feet and prolonged sleep-onset latency in vasospastic syndrome. Lancet. 2001; 358:125–126.

14. Flammer J, Orgül S, Costa VP, Orzalesi N, Krieglstein GK, Serra LM, et al. The impact of ocular blood flow in glaucoma. Prog Retin Eye Res. 2002; 21:359–393.

15. Ruijs AC, Jaquet JB, Daanen HA, Hovius SE. Cold intolerance of the hand measured by the CISS questionnaire in a normative study population. J Hand Surg Br. 2006; 31:533–536.

16. Jeong IK, King GL. New perspectives on diabetic vascular complications: the loss of endogenous protective factors induced by hyperglycemia. Diabetes Metab J. 2011; 35:8–11.

17. Mozaffarieh M, Fontana Gasio P, Schötzau A, Orgül S, Flammer J, Kräuchi K. Thermal discomfort with cold extremities in relation to age, gender, and body mass index in a random sample of a Swiss urban population. Popul Health Metr. 2010; 8:17.

18. Saner H, Würbel H, Mahler F, Flammer J, Gasser P. Microvasculatory evaluation of vasospastic syndromes. Adv Exp Med Biol. 1987; 220:215–218.

19. Wunderlich K, Zimmerman C, Gutmann H, Teuchner B, Flammer J, Drewe J. Vasospastic persons exhibit differential expression of ABC-transport proteins. Mol Vis. 2003; 9:756–761.
20. Kang S, Chung KH, Kim TY, Ahn S, Ha JW, Rim S, et al. The inhibitory effect of triflusal (Disgren)on the platelet aggregation in healthy volunteers: impedance method with the whole blood. Korean Circ J. 1998; 28:707–714.

21. Matías-Guiu J, Ferro JM, Alvarez-Sabín J, Torres F, Jiménez MD, Lago A, et al. Comparison of triflusal and aspirin for prevention of vascular events in patients after cerebral infarction: the TACIP Study: a randomized, double-blind, multicenter trial. Stroke. 2003; 34:840–848.

22. Cruz-Fernández JM, López-Bescós L, García-Dorado D, López García-Aranda V, Cabadés A, Martín-Jadraque L, et al. Randomized comparative trial of triflusal and aspirin following acute myocardial infarction. Eur Heart J. 2000; 21:457–465.

24. De La Cruz JP, Villalobos MA, García PJ, Smith-Agreda JM, Sánchez de la Cuesta F. Effects of triflusal and its main metabolite HTB on platelet interaction with subendothelium in healthy volunteers. Eur J Clin Pharmacol. 1995; 47:497–502.

25. de la Cruz JP, Mata JM, Sanchez de la Cuesta F. Triflusal vs aspirin on the inhibition of human platelet and vascular cyclooxygenase. Gen Pharmacol. 1992; 23:297–300.

26. García-Rafanell J, Ramis J, Gomez L, Forn J. Effect of triflusal and other salicylic acid derivatives on cyclic AMP levels in rat platelets. Arch Int Pharmacodyn Ther. 1986; 284:155–165.
27. Bayón Y, Alonso A, Sánchez Crespo M. 4-trifluoromethyl derivatives of salicylate, triflusal and its main metabolite 2-hydroxy-4-trifluoromethylbenzoic acid, are potent inhibitors of nuclear factor kappaB activation. Br J Pharmacol. 1999; 126:1359–1366.

28. Aceves M, Dueñas A, Gómez C, San Vicente E, Crespo MS, García-Rodríguez C. A new pharmacological effect of salicylates: inhibition of NFAT-dependent transcription. J Immunol. 2004; 173:5721–5729.

29. Sánchez de Miguel L, Casado S, Farré J, García-Durán M, Rico LA, Montón M, et al. Comparison of in vitro effects of triflusal and acetysalicylic acid on nitric oxide synthesis by human neutrophils. Eur J Pharmacol. 1998; 343:57–65.
Supplementary Material
Supplementary Fig. 1
Representative case. After 6 weeks of aspirin administration, the CISS score decreased and radial peak velocity increased when compared with the baseline values. An increase in ICG perfusion rate from the baseline values was also observed. After a 2-week wash out period, triflusal was administered for another 6 weeks. As expected, the CISS score was further decreased and PSV and ICG perfusion rate were increased even further. CISS, cold intolerance symptom severity; ICG, indocyanine green; PSV, peak systolic velocity.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download