Abstract
Purpose
To investigate predictors of progression to castration-resistant prostate cancer (CRPC) and cancer-specific mortality (CSM) in patients with metastatic prostate cancer (mPCa).
Materials and Methods
A retrospective analysis was performed on 440 consecutive treatment-naïve patients initially diagnosed with mPCa between August 2000 and June 2012. Patient age, body mass index (BMI), Gleason score, prostate-specific antigen (PSA), PSA nadir, American Joint Committee on Cancer stage, Visual Analogue Scale pain score, Eastern Cooperative Oncology Group performance score (ECOG PS), PSA response to hormone therapy, and metastatic sites were assessed. Cox-proportional hazards regression analyses were used to evaluate survivals and predictive variables of men with bone metastasis stratified according to the presence of pain, compared to men with visceral metastasis.
Results
Metastases were most often found in bone (75.4%), followed by lung (16.3%) and liver (8.3%) tissues. Bone metastasis, pain, and high BMI were associated with increased risks of progression to CRPC, and bone metastasis, pain, PSA nadir, and ECOG PS≥1 were significant predictors of CSM. During the median follow-up of 32.0 (interquartile range 14.7-55.9) months, patients with bone metastasis with pain and patients with both bone and visceral metastases showed the worst median progression to CRPC-free and cancer-specific survivals, followed by men with bone metastasis without pain. Patients with visceral metastasis had the best median survivals.
The prostate-specific antigen (PSA) era has seen decreases in risk and stage migration in prostate cancer (PCa).1 Even so, bone metastases still constitute approximately 3% of newly diagnosed patients, and 12% of patients without initial evidence are destined to develop bone metastasis during a median follow-up of 2.2 years.23 In advanced stage PCa, bone metastasis occurs in more than 80% of cases, with a corresponding high level of morbidity and a 5-year survival rate of 25%.4 In contrast, the natural history of visceral metastasis is poorly characterized. Although metastasis to the viscera was previously considered uncommon and clinically irrelevant, autopsy studies of men who died of PCa demonstrate visceral involvement in up to 66% of cases.5 Thus, the prognostic impact of visceral disease on survival deserves consideration.
Androgen deprivation therapy (ADT) is the first-line therapy for patients with metastatic PCa (mPCa); nevertheless, castration resistance is destined to emerge, conferring an increased risk of cancer-specific mortality (CSM).6 The heterogeneous natural history of mPCa progression to castration-resistant PCa (CRPC) and eventual CSM poses a challenge to treating clinician. The ability to identify risk factors of progression to CRPC and CSM in treatment-naïve patients with mPCa would represent an important advance in identifying the underlying mechanisms of CRPC development and disease progression. Such knowledge would facilitate early identification of those most likely to benefit from targeted therapy to prevent or delay progression. Intensive monitoring could optimize the likelihood of a successful intervention, and those at low risk of progression could avoid unnecessary testing.
A few population-based observational studies have reported on survival outcomes in patients with mPCa,78 and a number of predictive models have been developed based on variables pertaining to survival.910 However, to the best of our knowledge, data are limited regarding survival outcomes according to site of metastasis in treatment-naïve patients initially diagnosed with mPCa. In such patients, previous intervention would not confound survival analysis.
The aims of this study were to 1) explore the distribution of metastatic sites in treatment-naïve patients initially diagnosed with mPCa, 2) evaluate predictors pertaining to progression to CRPC and CSM, and 3) investigate how the site of metastasis and clinicopathological features may adversely affect survival outcomes.
A retrospective analysis was performed from a prospectively collected database of 440 consecutive treatment-naïve patients diagnosed with mPCa between August 2000 and June 2012. Patients were stratified into four groups: men with bone metastasis only without pain; the same but with pain; men with visceral metastasis only; and men with both bone and visceral metastases. The presence of enlarged regional pelvic lymph nodes was excluded from the definition of metastasis. PCa staging was determined according to the 7th American Joint Committee on Cancer (AJCC) TNM system, with the definition of distant metastasis based on either demonstrable metastatic deposits on imaging (bone scan, computerized tomography, magnetic resonance imaging, or positron emission tomography) or pathologic confirmation of PCa from tissue outside the prostatic fossa. Patients were excluded from analysis if they met the following criteria: 1) incomplete clinical data; 2) lost to follow-up; 3) previous treatment targeted at PCa, including bone-sparing agents; and 4) unknown cause of death. Patients had regular serum PSA measurements and imaging follow-up at least six months apart. All patients received ADT consisting of luteinizing hormone-releasing hormone agonists with or without anti-androgens, with docetaxel being the routinely administered first-line cytotoxic chemotherapy following progression to CRPC. The initiation and regimens of ADT and cytotoxic chemotherapy were based on physician discretion and patient preference. This study was approved by the institutional ethics committee after review of the protocol and procedures employed (3-2014-0112).
Covariates consisted of patient age, body mass index (BMI), hemoglobin, alkaline phosphatase, Gleason score, PSA and PSA nadir levels, AJCC stage, Visual Analogue Scale (VAS) pain score, Eastern Cooperative Oncology Group performance score (ECOG PS), Charlson Comorbidity Index (CCI), CRPC status, time to CRPC progression, and the site of metastasis. Pain was defined as a VAS pain score ≥1, which included conditions requiring anesthetics, palliative radiation therapy, or surgery. Pathologic outcomes were based on previous reports confirm-ed by a single genitourinary pathologist at our institution. CRPC was defined as progression of disease or elevation of serum PSA using the Prostate Cancer Working Group 2 (PCWG2) criteria, 11 with progression to CRPC-free interval defined as the time from the date of the first diagnosis of mPCa to the date of CRPC diagnosis. The CSM interval was defined as the interval from the first diagnosis of mPCa to the date of death from PCa. For all patients, the status of survival and cause of death were investigated through the National Cancer Registry Database or by institutional electronic medical records.
The primary endpoint was to identify prognostic factors associated with progression to CRPC and CSM. The secondary endpoint was to investigate how metastatic site and the clinicopathological feature of pain affected survival outcome.
Demographic characteristics of patients and tumors were compared using descriptive statistics. Appropriate comparative tests, such as the Student's t-test and χ2-test, were used to compare continuous and categorical variables. Kaplan-Meier curves were used to estimate survival of men with bone metastasis stratified by the presence of pain compared to men with visceral metastasis. Univariate and multivariate analyses were performed according to Cox-proportional hazards regression models in order to adjust for potential confounders in predicting survival. Variables considered potential predictors for multivariate modeling were selected by univariate analysis. Statistical analysis was performed using SPSS version 18 (SPSS Inc., Chicago, IL, USA). All tests were two-sided, with statistical significance set at p<0.05.
Among 440 initially diagnosed treatment-naïve patients with mPCa, 248 (56.4%), 158 (35.9%), and 34 (7.7%) patients were diagnosed with bone metastasis only, visceral metastasis only, and both bone and visceral metastases, respectively. Clinicopathologic features of each group are presented in Table 1. Patients with bone metastasis were more likely to be diagnosed at a higher stage and grade of PCa, and to have higher PSA and PSA nadir levels, VAS pain score (≥1), and ECOG PS (≥1), compared to men with visceral metastasis. The groups were comparable in terms of age, BMI, and distribution of CCI (≥4). Metastases were most often to bone (75.4%), followed by lung (16.3%) and liver (8.3%).
Multivariate analyses revealed bone metastasis [hazard ratio (HR)=2.790; 95% confidence interval (CI) 1.616-4.819], presence of pain (HR=1.883; 95% CI 1.120-3.168), and high BMI ( HR=1.119; 95% CI 1.046-1.196) to be associated with an increased risk of progression to CRPC. However, tumor grade and stage, initial PSA level, and time to PSA nadir following ADT did not predict emergence of CRPC (Table 2). Bone metastasis (HR=1.758; 95% CI 1.152-2.683), presence of pain (HR=1.861; 95% CI 1.177-2.910), ECOG PS ≥1 (HR=1.006; 95% CI 1.002-1.011), and PSA nadir (HR=1.002; 95% CI 1.001-1.002) were significant predictors of CSM. Of note, initial PSA level at diagnosis and progression to CRPC were not associated with CSM (Table 3).
Survival results as of March 2014 were used in this analysis. With pain revealed as a predictor for survival in the multivariable analysis, patients with bone metastasis were stratified according to the presence of pain and compared to patients with visceral metastasis and those with both bone and visceral metastases. The median intervals from initial diagnosis of metastasis to CRPC progression and CSM, as well as the survival outcome of each group, are presented in Table 4 and Fig. 1. Due to clinicopathological heterogeneity across subgroups, which may have confounded our ability to determine the influence of bone metastasis and the presence of pain on survival outcomes, we evaluated the comparative survival of patients after adjustment for covariates considered potential predictors by the Cox-proportional hazards analysis. For all study endpoints, patients with bone metastasis accompanied by pain and patients with both bone and visceral metastases had the worst median survivals, followed by men with bone metastasis without pain. Patients with visceral metastasis had the best median survivals.
In this retrospective analysis of treatment-naïve patients initially diagnosed with mPCa, the site of metastasis and the presence of pain were independent predictors of both progression to CRPC and CSM. Patients with bone metastasis showed worse progression to CRPC-free and cancer-specific survival rates than men with visceral metastasis. Among patients with bone metastasis, men without pain demonstrated better survival outcomes than men with pain, who exhibited comparable outcomes with men with both bone and visceral metastases.
The tumor microenvironment is a rich source of variable soluble factors that play a critical role in tumor aggressiveness and androgen sensitivity, a key feature of PCa cells.12 Bone is the single most dominant site of metastasis and is a metastatic site in 90% of patients with mPCa.13 The propensity of PCa to preferentially metastasize to bone may be explained by the fact that the bone microenvironment provides a fertile setting for the growth and aggressive development of PCa cells.1415 Metastasis to the bone may contribute to the emergence of CRPC and disease progression, and several studies have reported a number of factors in the bone microenvironment that may facilitate castration resistance, namely, epidermal growth factor, insulin-like growth factor (IGF), keratinocyte growth factor (FGF-7), and interleukin (IL)-6.161718 Moreover, factors secreted from the local bone environment as a result of osteoclastic bone resorption, namely IGF-I, transforming growth factor-β, bone morphogenic proteins, parathyroid hormone-related protein, IL-1, and IL-6, have been shown to aid PCa cells in escaping apoptosis, developing treatment-resistant phenotypes, and continuing to proliferate and grow.192021 An in vitro experimental study found that co-culture of PCa cells with bone stromal cell lines induces PCa cells to become androgen resistant.22 Our results are consistent with a population-based study of metastatic CRPC patients in which survival of men with visceral metastasis was affected by the degree of bone involvement.23 These observations imply that the molecular basis for the development of androgen resistance is linked to fundamental changes in the bone microenvironment, which may provide an explanation for the increased overall survival seen in patients treated by bone-targeted radiopharmaceuticals that exert a potent effect on both PCa cells and host cells within the bone.1524
We observed that bone metastasis is associated with an increased risk of progression to CRPC and CSM and that this association is stronger for bone metastasis complicated with pain. Pain, pathological fracture, spinal cord compression, and bone surgery all represent a spectrum of skeletal-related events that have been shown to predict poor prognosis.2 Our results are consistent with observations of the US Surveillance, Epidemiology, and End Results (SEER)-Medicare and Danish National Patient Registry-based studies, which reported similar results in patients with bone mPCa.225 Although the underlying mechanism is unclear, an increased mechanical and chemical stimulation of periosteal or endosteal pain receptors resulting from increased overall disease burden may be pertinent.26 Unfortunately, we failed to detect any association between the degree of pain and the extent of bone metastasis in our cohort. Gandaglia, et al.27 recently reported outcomes of patients from the SEER database in which men with visceral metastasis showed inferior overall survival to that of men with bone metastasis. Of note, the inferior outcome was not significant for CSM. While the underlying mechanism of disparate survival outcome according to metastatic site observed between studies is unclear, we emphasize that retrospective studies should be cautiously interpreted within their limitations. Nonetheless, based on our results, we suggest pain as a valuable surrogate marker for survival in patients with bone mPCa, and that in turn, may be of direct relevance for early identification of poor survival. Palliation of symptoms may provide opportunities for a favorable clinical outcome.
PSA nadir level following ADT has been used as a useful prognosticator of disease progression and survival in various disease settings.28 Our results are in accordance to previous studies that have observed significant associations between PSA nadir and progression to CRPC.29 Moreover, we observed that obesity is associated with an increased risk of progression to CRPC. It has been widely reported that obese patients have higher risks of PCa-related death and disease progression.30 Although the underlying mechanism thereof is not fully understood, obesity-associated leptin and adiponectin have been suggested to adversely affect disease progression by promoting angiogenesis and PCa cell growth, respectively.31
Our study serves to inform clinical practice by highlighting that the prognosis of patients diagnosed of mPCa depends on multiple patient factors. The identification of clinical surrogates that capture early progression to CRPC and survival would be significant for several reasons. The natural history of men with initially diagnosed mPCa is heterogeneous and difficult to estimate using exclusively "classic" prognostic factors, namely, PSA kinetics, stage, and grade of the disease. Our results may refine prognosis and allow risk stratification of patients with mPCa at an early stage of diagnosis. Also, further evidence regarding the inter-relationship between PCa cells and bone microenvironment shifts the paradigm for understanding PCa growth and development of CRPC in bone, which may lead to development of therapies that target not only PCa cells but also supporting cells of the microenvironment.14
The strengths of the current study include the incorporation of detailed clinicopathologic data, information on treatment, comorbidities other than cancer, and performance status information that was available for each patient. Moreover, a single institutional cohort may justify the uniformity of the data. At the same time, we acknowledge several limitations: first, our study is limited by its observational, retrospective design, and results should be interpreted accordingly. Due to the heterogeneity of this population, there was a lack of standard therapeutic approach, and a physician and patient preference existed regarding the implementation of a specific treatment. Second, a subset of patients had evidence of bone metastasis based on technetium-99m bone scans, which are limited by inaccuracies and uncertainties arising from lack of specificity and inter-observer variability.32 Third, the prognostic factors that we identified were determined at the time of metastasis, and not before. Thus, the results may not be generalizable to evaluating risk for the average patient at the time of PCa diagnosis or after a certain definitive treatment.
This observational study uncovered novel findings on the patterns of metastatic spread and presence of pain, which may impact prognosis differently in patients initially diagnosed with mPCa. Bone seems to provide a crucial environment in the development of CRPC and CSM, implying that bone-targeted therapies for men at high risk for bone metastasis may potentially delay progression to CRPC and reduce risk of CSM. Further elucidation of the complex molecular interactions between PCa cells and the bone microenvironment may open new avenues for treatment.
Figures and Tables
 | Fig. 1Comparative survival curves of patients with metastatic prostate cancer for (A) progression to castration-resistant prostate cancer (CRPC)-free survival and (B) cancer-specific survival. |
Table 1
Clinicopathological Features of 440 Patients with Metastatic Prostate Cancer According to Site of Metastasis

Table 2
Predictors of Progression to Castration-Resistant Disease in Patients with Metastatic Prostate Cancer

Table 3
Predictors of Cancer-Specific Death in Patients with Metastatic Prostate Cancer
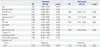
Table 4
Survival Outcomes of 440 Patients with Metastatic Prostate Cancer, Stratified According to Site of Metastasis and Presence of Pain

References
1. Mikel Hubanks J, Boorjian SA, Frank I, Gettman MT, Houston Thompson R, Rangel LJ, et al. The presence of extracapsular extension is associated with an increased risk of death from prostate cancer after radical prostatectomy for patients with seminal vesicle invasion and negative lymph nodes. Urol Oncol. 2014; 32:26.

2. Nørgaard M, Jensen AØ, Jacobsen JB, Cetin K, Fryzek JP, Sørensen HT. Skeletal related events, bone metastasis and survival of prostate cancer: a population based cohort study in Denmark (1999 to 2007). J Urol. 2010; 184:162–167.
3. Coleman RE. Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin Cancer Res. 2006; 12(20 Pt 2):6243s–6249s.

4. Sturge J, Caley MP, Waxman J. Bone metastasis in prostate cancer: emerging therapeutic strategies. Nat Rev Clin Oncol. 2011; 8:357–368.

5. Shah RB, Mehra R, Chinnaiyan AM, Shen R, Ghosh D, Zhou M, et al. Androgen-independent prostate cancer is a heterogeneous group of diseases: lessons from a rapid autopsy program. Cancer Res. 2004; 64:9209–9216.

6. Crawford ED, Eisenberger MA, McLeod DG, Spaulding JT, Benson R, Dorr FA, et al. A controlled trial of leuprolide with and without flutamide in prostatic carcinoma. N Engl J Med. 1989; 321:419–424.

7. Ryan CJ, Elkin EP, Cowan J, Carroll PR. Initial treatment patterns and outcome of contemporary prostate cancer patients with bone metastases at initial presentation: data from CaPSURE. Cancer. 2007; 110:81–86.

8. Cooperberg MR, Broering JM, Carroll PR. Risk assessment for prostate cancer metastasis and mortality at the time of diagnosis. J Natl Cancer Inst. 2009; 101:878–887.

9. Chodak GW, Vogelzang NJ, Caplan RJ, Soloway M, Smith JA. Independent prognostic factors in patients with metastatic (stage D2) prostate cancer. The Zoladex Study Group. JAMA. 1991; 265:618–621.

10. Halabi S, Small EJ, Kantoff PW, Kattan MW, Kaplan EB, Dawson NA, et al. Prognostic model for predicting survival in men with hormone-refractory metastatic prostate cancer. J Clin Oncol. 2003; 21:1232–1237.

11. Scher HI, Halabi S, Tannock I, Morris M, Sternberg CN, Carducci MA, et al. Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: recommendations of the Prostate Cancer Clinical Trials Working Group. J Clin Oncol. 2008; 26:1148–1159.

12. Logothetis CJ, Navone NM, Lin SH. Understanding the biology of bone metastases: key to the effective treatment of prostate cancer. Clin Cancer Res. 2008; 14:1599–1602.

13. Hess KR, Varadhachary GR, Taylor SH, Wei W, Raber MN, Lenzi R, et al. Metastatic patterns in adenocarcinoma. Cancer. 2006; 106:1624–1633.

14. Loberg RD, Logothetis CJ, Keller ET, Pienta KJ. Pathogenesis and treatment of prostate cancer bone metastases: targeting the lethal phenotype. J Clin Oncol. 2005; 23:8232–8241.

15. Seruga B, Ocana A, Tannock IF. Drug resistance in metastatic castration-resistant prostate cancer. Nat Rev Clin Oncol. 2011; 8:12–23.

16. Efstathiou E, Logothetis CJ. A new therapy paradigm for prostate cancer founded on clinical observations. Clin Cancer Res. 2010; 16:1100–1107.

17. laszczyk N, Masri BA, Mawji NR, Ueda T, McAlinden G, Duncan CP, et al. Osteoblast-derived factors induce androgen-independent proliferation and expression of prostate-specific antigen in human prostate cancer cells. Clin Cancer Res. 2004; 10:1860–1869.

18. Corn PG. The tumor microenvironment in prostate cancer: elucidating molecular pathways for therapy development. Cancer Manag Res. 2012; 4:183–193.

19. Achbarou A, Kaiser S, Tremblay G, Ste-Marie LG, Brodt P, Goltzman D, et al. Urokinase overproduction results in increased skeletal metastasis by prostate cancer cells in vivo. Cancer Res. 1994; 54:2372–2377.
20. Koutsilieris M, Rabbani SA, Goltzman D. Effects of human prostatic mitogens on rat bone cells and fibroblasts. J Endocrinol. 1987; 115:447–454.

21. Nelson JB, Hedican SP, George DJ, Reddi AH, Piantadosi S, Eisenberger MA, et al. Identification of endothelin-1 in the pathophysiology of metastatic adenocarcinoma of the prostate. Nat Med. 1995; 1:944–949.

22. Lee GT, Kang DI, Ha YS, Jung YS, Chung J, Min K, et al. Prostate cancer bone metastases acquire resistance to androgen deprivation via WNT5A-mediated BMP-6 induction. Br J Cancer. 2014; 110:1634–1644.

23. Pezaro CJ, Omlin A, Lorente D, Nava Rodrigues D, Ferraldeschi R, Bianchini D, et al. Visceral disease in castration-resistant prostate cancer. Eur Urol. 2014; 65:270–273.
24. Parker C, Nilsson S, Heinrich D, Helle SI, O'Sullivan JM, Fosså SD, et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med. 2013; 369:213–223.

25. Sathiakumar N, Delzell E, Morrisey MA, Falkson C, Yong M, Chia V, et al. Mortality following bone metastasis and skeletal-related events among men with prostate cancer: a population-based analysis of US Medicare beneficiaries, 1999-2006. Prostate Cancer Prostatic Dis. 2011; 14:177–183.

26. Msaouel P, Nandikolla G, Pneumaticos SG, Koutsilieris M. Bone microenvironment-targeted manipulations for the treatment of osteoblastic metastasis in castration-resistant prostate cancer. Expert Opin Investig Drugs. 2013; 22:1385–1400.

27. Gandaglia G, Karakiewicz PI, Briganti A, Passoni NM, Schiffmann J, Trudeau V, et al. Impact of the Site of Metastases on Survival in Patients with Metastatic Prostate Cancer. Eur Urol. 2014; Aug. 6. [Epub]. DOI: 10.1016/j.eururo.2014.07.020.

28. Hong SY, Cho DS, Kim SI, Ahn HS, Kim SJ. Prostate-specific antigen nadir and time to prostate-specific antigen nadir following maximal androgen blockade independently predict prognosis in patients with metastatic prostate cancer. Korean J Urol. 2012; 53:607–613.

29. Morote J, Esquena S, Abascal JM, Trilla E, Cecchini L, Raventós CX, et al. Usefulness of prostate-specific antigen nadir as predictor of androgen-independent progression of metastatic prostate cancer. Int J Biol Markers. 2005; 20:209–216.

30. Park JM, Nam JS, Na W, Oh JJ, Lee S, Hong SK, et al. Prognostic value of body mass index in Korean patients with castration-resistant prostate cancer. Korean J Urol. 2012; 53:761–765.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download