This article has been
cited by other articles in ScienceCentral.
Abstract
Behçet's disease (BD) involves multisystem vasculitis of unknown origin. Ocular manifestations of BD mostly include bilateral panuveitis and retinal vasculitis, which are very challenging to treat. Interferon alfa-2a (IFN) has been recently introduced for treating refractory Behçet uveitis, mainly in Germany and Turkey. Nonetheless, there is so far no consensus about the ideal treatment regimen of IFN for Behçet uveitis. We report our experience of IFN treatment in five Korean BD patients with refractory uveitis. All patients complained of oral ulcers; one patient had a positive pathergy test and 2 showed the presence of HLA-B51. Immunosuppressive agents used prior to IFN treatment included cyclosporine and methotrexate. The IFN treatment was commenced with a dose of 6-9 MIU/day for 7 days, adjusted according to individual ocular manifestations, tapered down to 3 MIU three times in a week, and then discontinued. All patients showed positive response to IFN treatment; 50% of them showed complete response without additional major ocular inflammation during the follow-up period. Other BD symptoms also improved after IFN treatment in most cases. After treatment, the relapse rate and the required dose of oral corticosteroid were decreased in most cases, showing a significant steroid-sparing effect. However, the visual acuity was not improved in most cases due to irreversible macular sequelae. Despite the small sample size of this study, we suggest that, in Korean patients, IFN is an effective treatment modality for BD uveitis as was observed in German and Turkish patients.
Keywords: Behçet's disease, interferon alfa-2a, uveitis
INTRODUCTION
Behçet's disease (BD) involves multisystem vasculitis of unknown origin, mainly characterized by recurrent oral ulcers, genital ulcers, ocular lesions, and skin lesions.
1 Ocular manifestations of BD mostly include bilateral panuveitis and retinal vasculitis, with a chronic repetitive relapsing-remitting course. According to a Korean nationwide retrospective study, approximately 6.4% (24 out of 376) of Korean BD patients experience blindness as sequelae to Behçet uveitis.
23 Refractory Behçet uveitis is very challenging to treat, and interferon alfa-2a (IFN) has been recently introduced for treating refractory Behçet uveitis patients, mainly in Germany and Turkey.
45 However, there is no consensus regarding the ideal IFN treatment regimen for BD. In this study, we report our experience of the treatment with IFN in five Korean BD patients with refractory uveitis.
CASE REPORT
From 1995 to 2014, five BD patients (4 men and 1 woman) were treated with IFN for refractory uveitis in our institute. This study was approved by the Institutional Review Board of Ajou University Hospital (AJIRB-MED-EXP-14-178). The clinical characteristics and treatment efficacy of each patient are summarized in
Table 1. The age of patients rang-ed between 30 and 47 years, with BD manifesting at a mean age of 31.2 years (range: 26 to 37 years). All patients complained of oral ulcers; one patient (case 2) showed a positive pathergy test, and 2 patients showed the presence of HLA-B51. Immunosuppressive agents used prior to IFN treatment included cyclosporine and methotrexate.
The regimen for IFN treatment was as follows, using previously described dosing schedule:
4 IFN treatment was initiated with a dose of 6-9 MIU/day for 7 days, adjusted according to individual ocular manifestations, tapered down to 3 MIU three times in a week, and then discontinued (
Fig. 1). During the first 7 days of IFN treatment, concomitant oral steroid (prednisolone 10 mg/d) was prescribed to each patient.
4 Other immunosuppressive agents were discontinued when IFN was initiated. Acetaminophen was given on day 1-3 in case of presenting flu-like symptom due to IFN. All patients showed positive response to IFN treatment (
Table 1), and their ocular inflammations subsided during the adjusting period of the daily dose (
Fig. 2). Other BD symptoms improved gradually in most cases, also later in the maintenance period. Sixty percent of patients showed complete response (CR) without additional major ocular inflammation observed during the follow-up period. Symptom-free period was defined as the time period until the next recurrence of the ocular inflammation. Case 2 and 3 presented mild ocular inflammation after 3 months and 1 month, respectively, from the end of IFN treatment, which was controlled with topical corticosteroid agent. Two patients (case 4 and 5) showed partial response, which was defined as at least one recurrence during IFN treatment period and/or the additional use of low-dose oral corticosteroid or other immunosuppressive agent after IFN treatment (
Table 1). They also showed only minimal improvement of other BD symptoms compared with other patients who showed CR to IFN. Methotrexate, azathioprine, and oral corticosteroid were additionally used after finishing IFN treatment in case 5.
After IFN treatment, the relapse rate and the required dose of oral corticosteroids were decreased in most cases, showing a significant steroid-sparing effect. However, the visual acuity was not improved in most cases. Common adverse effects included flu-like symptoms and myalgia, which were observed in all cases, however, they were resolved within 1 to 2 weeks without discontinuing IFN treatment. Other observed adverse effects were gastrointestinal symptoms (nausea or vomiting) and hair loss, which are consistent with the previous findings.
DISCUSSION
IFN was first used for Behçet uveitis in 1994, and its excellent treatment efficacy has previously been reported.
67 The response rate has been shown to range from 80% to 90%, and IFN is considered effective for improving vitreous haze, retinal vasculitis, and macular edema.
478
The mode of action of IFN in BD is obscure. It has been suggested that the immunomodulatory effect of IFN may be attributable to the reduction of HLA class I antigen expression on monocytes and the inhibition of the proliferation of natural killer and γδT cells, or an immunosuppressive effect that directly suppresses vasculitis.
49
The beneficial effects of IFN treatment, as observed in this case series, include lower relapse rate, oral steroid-sparing effect, and simultaneous improvement of other systemic BD symptoms. In contrast to previous studies, however, the visual acuity was not dramatically improved in our patients, and we believe that irreversible macular sequelae, such as macular holes or retinal ischemia, was the cause of the unchanged or even worse final vision in some patients despite well-controlled ocular inflammation.
There is no consensus so far in terms of the dosing regimen, but it might be individualized depending on the severity of ocular inflammation. The range of duration of IFN use varies between 3 to 58 months according to previous studies.
24578 We have individualized the dose and duration of IFN according to patient's ocular inflammation, however, the duration of IFN seemed shorter compared with other reports. It might be speculated that the relative short duration of using IFN resulted in the partial responsiveness on two patients.
Despite the small sample size of this study, we suggest that IFN would be effective in the treatment of BD uveitis in Korean patients, and that also improves other BD symptoms additionally. However, considering the fact that irreversible macular sequalae result in severe visual impairment, one should consider to initiate IFN for the patient with refractory uveitis who shows no response to at least one immunosuppressive agent and co-existence of macular edema and/or retinal vasculitis.
10 Especially, in terms of macular edema, recurrent episode can lead to poor visual recovery. The earlier the intervention, the better visual acuity could be preserved. Further large scale study to investigate the ideal treatment starting time to prevent irreversible change would be needed.
In conclusion, IFN is effective and well-tolerated for Korean BD patients with refractory uveitis. Nevertheless, further randomized controlled trials are needed to compare the efficacy of IFN to that of other immunosuppressive agents.
Figures and Tables
Fig. 1
Flow chart for interferon alfa-2a (IFN) dosage. BD, Behçet's disease.

Fig. 2
Fundus photography and fluorescein angiography of one patient (Case 4) with Behçet uveitis. (A) Before vascular occlusion (at the 4-month follow-up): retinal vasculitis of the left eye. Fluorescein angiography (FA) shows diffuse leakage of fluorescein dye from the whole retinal vasculature and the optic disc. (B) Retinal vascular occlusion (before interferon alfa-2a treatment, at the 8-month follow-up): branch retinal vein occlusion with intraretinal edema of the left eye. FA shows blocked vascular filling at the inferotemporal venous branch. (C) After interferon alfa-2a treatment (at the 14-month follow-up): tortuous retinal vessels at the inferotemporal area, with improved retinal hemorrhage and edema. FA shows reperfusion; however, narrow inferotemporal venous branch with wide peripheral non-perfusion areas are also noted.
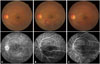
Table 1
The Clinical Characteristics and Treatment Efficacy of the Patients

|
Case 1 (M/47) |
Case 2 (M/47) |
Case 3 (M/46) |
Case 4 (M/42) |
Case 5 (F/30) |
|
BD manifestation |
Orogenital ulcer, EN, ocular lesion, arthralgia |
Orogenital ulcer, EN, ocular lesion, epididymitis |
Orogenital ulcer, ocular lesion, arthralgia |
Oral ulcer, ocular lesion |
Oral ulcer, EN, ocular lesion |
|
First BD Sx |
Oral ulcer, EN |
Oral ulcer |
Oral ulcer, ocular lesion |
Oral ulcer |
Oral ulcer, EN |
|
Age of BD onset |
33 yrs |
29 yrs |
30 yrs |
37 yrs |
26 yrs |
|
HLA-B51 |
(+) |
(+) |
(-) |
(-) |
(-) |
|
Ocular diagnosis |
Panuveitis (OU) |
Panuveitis (OU) |
Panuveitis (OU) |
Panuveitis (OU) |
Panuveitis (OD) |
|
Retinal vasculitis (OS) |
Retinal vasculitis (OS) |
Macular hole (OS) |
Macular edema (OU) |
Retinal vasculitis (OD) |
|
Macular hole (OU) |
|
Retinal detachment (OS) |
Retinal vasculitis (OS) |
|
|
Retinal detachment (OS) |
|
|
Branch retinal vein occlusion (OS) |
|
|
Previous immunosuppressive agents |
Cyclosporine |
Cyclosporine |
Cyclosporine |
Cyclosporine, methotrexate |
Cyclosporine |
|
IFN Tx (before → after IFN) |
|
|
|
|
|
|
Initial dosage |
6 MIU/day |
6 MIU/day |
9 MIU/day |
6 MIU/day |
6 MIU/day |
|
Duration of Tx |
21 wks |
34 wks |
28 wks |
24 wks |
42 wks |
|
Dose of oral corticosteroid |
l-d 32 mg/d → 0 mg/d |
l-d 24 mg/d → 0 mg/d |
l-d 24-8 mg/d → 0 mg/d |
p-l 60-20 mg/d → 5 mg/d |
l-d 48-24 mg/d → 5 mg/d (prn) |
|
Concurrent other Tx |
None |
None |
None |
IVDI (Recurred inflammation once during Tx) |
PSTI (recurred inflammation once during Tx) |
|
No. of ocular Sx relapses |
1.27/yr → 0/yr |
0.5/yr → 1.5/yr |
0.93/yr → 0/yr |
3.33/yr → 1/yr |
2.67/yr → 2/yr |
|
Visual acuity (OD, OS) |
FC/2 m, FC/3 m→ FC/30 cm, 0.05 |
HM, HM→ FC/50 cm, 0.2 |
LP (-), LP (-) → ND |
1.0, 0.1 → 1.0, FC/30 cm |
FC/30 cm, 1.0 → 0.25-2, 1.0 |
|
Improvement of other BD Sx after IFN Tx |
Orogenital ulcer, arthralgia: none |
Genital ulcer: none |
Genital ulcer: none |
Oral ulcer: decreased |
No improvement |
|
EN: decreased |
Oral ulcer, EN: decreased |
Oral ulcer, arthralgia: decreased |
|
Symptom-free period after Tx |
102 months |
3 months |
1 month |
5 months |
1.5 month |
|
Overall follow-up period after Tx |
102 months |
74 months |
91 months |
26 months |
12 months |
|
Adverse event |
Flu-like Sx, myalgia, nausea |
General weakness |
Flu-like Sx, myalgia, nausea, diarrhea |
Flu-like Sx, myalgia, hair loss |
Flu-like Sx, nausea, vomiting |
|
IFN response |
CR |
CR |
CR |
PR |
PR |
ACKNOWLEDGEMENTS
This study was supported by a grant of the Korea Healthcare Technology R&D Project, Ministry of Health & Welfare, Republic of Korea (A080588).
References
1. Sakane T, Takeno M, Suzuki N, Inaba G. Behçet's disease. N Engl J Med. 1999; 341:1284–1291.

2. Bodaghi B, Gendron G, Wechsler B, Terrada C, Cassoux N, Huong du LT, et al. Efficacy of interferon alpha in the treatment of refractory and sight threatening uveitis: a retrospective monocentric study of 45 patients. Br J Ophthalmol. 2007; 91:335–339.

3. Na S, Park J, Cho S, Cho S, Bang D, Rho J, et al. Epidemiological and clinical characteristics of Behcet's disease in Korea - using a clinical database for patients' registry. In : 15th International conference on Behcet's disease 2012; Yokohama, Japan.
4. Kötter I, Zierhut M, Eckstein AK, Vonthein R, Ness T, Günaydin I, et al. Human recombinant interferon alfa-2a for the treatment of Behçet's disease with sight threatening posterior or panuveitis. Br J Ophthalmol. 2003; 87:423–431.
5. Onal S, Kazokoglu H, Koc A, Akman M, Bavbek T, Direskeneli H, et al. Long-term efficacy and safety of low-dose and dose-escalating interferon alfa-2a therapy in refractory Behçet uveitis. Arch Ophthalmol. 2011; 129:288–294.

6. Feron EJ, Rothova A, van Hagen PM, Baarsma GS, Suttorp-Schulten MS. Interferon-alpha 2b for refractory ocular Behçet's disease. Lancet. 1994; 343:1428.
7. Evereklioglu C. Ocular Behçet disease: current therapeutic approaches. Curr Opin Ophthalmol. 2011; 22:508–516.
8. Sobaci G, Erdem U, Durukan AH, Erdurman C, Bayer A, Köksal S, et al. Safety and effectiveness of interferon alpha-2a in treatment of patients with Behçet's uveitis refractory to conventional treatments. Ophthalmology. 2010; 117:1430–1435.

9. Treusch M, Vonthein R, Baur M, Günaydin I, Koch S, Stübiger N, et al. Influence of human recombinant interferon-alpha2a (rhIFN-alpha2a) on altered lymphocyte subpopulations and monocytes in Behcet's disease. Rheumatology (Oxford). 2004; 43:1275–1282.

10. Zierhut M, Abu El-Asrar AM, Bodaghi B, Tugal-Tutkun I. Therapy of ocular Behçet disease. Ocul Immunol Inflamm. 2014; 22:64–76.







 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download