Abstract
Purpose
Materials and Methods
Results
Figures and Tables
Fig. 1
An overlayed circle of diameter 5.5 mm was centered on the fovea to define the posterior pole region approximating the superior and inferior temporal vascular arcades in a patient with Behçet retinal vasculitis (the outer circle). The macular region was delineated with another overlayed circle of diameter 1.5 mm centered on the fovea (the inner circle).
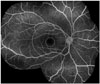
Fig. 2
Fluorescein angiogram showing focal leakage (A, arrow pointing at a focal leakage site at the infero-temporal quadrant) and diffuse vascular staining and leakage (B) in Behçet retinal vasculitis.
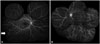
Fig. 3
Fluorescein angiograms demonstrating different grades of macular leakage in Behçet retinal vasculitis. Grade 1, incomplete perifoveal hyperfluorescence (A); grade 2, mild 360° hyperfluorescence (B); grade 3, moderate 360° hyperfluorescence with the hyperfluorescent area being approximately 1 disc diameter across (C); grade 4, severe 360° hyperfluorescence with the hyperfluorescent area being approximately 1.5 disc diameters across (D).
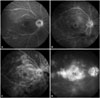
Fig. 4
Fluorescein angiograms demonstrating different degrees of retinal vascular leakage in Behçet retinal vasculitis. Mild, staining of vessels with minimal leakage (A); moderate, more intense leakage with a distinct vascular margin (B); severe, even greater leakage with blurring of the large vessel margins (C).
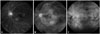
Fig. 5
Fluorescein angiograms of Behçet retinal vasculitis demonstrating varying degrees of optic disc hyperfluorescence. Partial (A); diffuse with clear disc margin (B); diffuse with blurring of the disc margin (C).
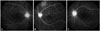
Table 1
Fluorescein Angiography Findings in Behçet Retinal Vasculitis

logMAR, logarithm of the minimum angle of resolution; VA, visual acuity; NVE, neovascularization elsewhere; NVD, neovascularization at disc; BRVO, branch retinal vein occlusion.
*Peripheral vasculitis refers to eyes with vascular leakage confined to the periphery (i.e., no involvement of the posterior pole).
†Vasculitis involving the posterior pole refers to eyes with vascular leakage that involves the posterior pole. All of these eyes also had vascular leakage in the periphery.
‡Macular subtype had involvement of the posterior pole region that included the central 1.5 mm area of the macula.
§Extramacular subtype showed involvement of the posterior pole but no evidence of macular leakage.
∥Comparison between the macular and extramacular subtypes.
¶Comparison between peripheral vasculitis and posterior pole-involved vasculitis.
**Pearson chi-square test (Fisher's exact test).
††Independent Student's t-test or Mann-Whitney U test.
‡‡Mantel-Haenszel chi-square test for trend analysis.
§§Follow-up VA at 2 year time point within a 3 month window; p-value<0.05 is considered statistically significant (indicated in italic).
Table 2
Factors Associated with Visual Acuity in Posterior Pole-Involved Retinal Vasculitis by Univariable Analysis in Behçet Retinal Vasculitis

Table 3
Factors Associated with Visual Acuity in Posterior Pole-Involved Retinal Vasculitis by Multivariable Analysis in Behçet Retinal Vasculitis

Adjusted R2=0.408. A multivariable regression analysis with stepwise variable selection was used.
*The magnitude of angiographic parameters was taken into account in the analysis.
†Anterior chamber cell: 0, 1+, 2+, 3+, or more.
‡Vitreous cell: 0, 1+, 2+, 3+, or more.
§Statistically significant p-value (<0.05) is indicated in italic.
Table 4
Changes in Vascular Leakage and Visual Acuity during Follow Up in Behçet Retinal Vasculitis

VA, visual acuity.
Follow up=time interval between the baseline and the closest-to-two year follow-up visit. p-value<0.05 is considered statistically significant.
*Retinal vascular leakage, optic disc hyperfluorescence, and macular leakage were compared collectively between the initial and at 2 year time point within a 3 months window. Worsened=there was an increase in the magnitude of the overall vascular leakage, No change=there was no discernible difference in the degree of leakage, Improved=there was a decrease in the magnitude of the overall vascular leakage.
†For a change in VA to be considered a gain, there had to be an improvement of at least three Snellen chart lines between the initial VA and VA at 2 year time point within a 3 months window. A drop of at least three Snellen chart lines was considered a loss of VA. A change in VA of fewer than three lines was regarded as no change.
Table 5
Systemic Treatment Summary in Patients with Behçet Retinal Vasculitis





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download