Abstract
Purpose
To determine the prevalence and characteristics of neuropathic pain (NP) in patients with lumbar spinal stenosis (LSS) according to subgroup analysis of symptoms.
Materials and Methods
We prospectively enrolled subjects with LSS (n=86) who were scheduled to undergo spinal surgery. The patients were divided into two groups according to a chief complaint of radicular pain or neurogenic claudication. We measured patient's pain score using the visual analog scale (VAS), Oswestry Disability Index (ODI) and Leads Assessment of Neuropathic Symptoms and Signs (LANSS). According to LANSS value, the prevalence of NP component pain in patients with LSS was assessed. Statistical analysis was performed to find the relationship between LANSS scores and the other scores.
Results
From our sample of 86 patients, 31 (36.0%) had a NP component, with 24 (63.4%) in the radicular pain group having NP. However, only seven patients (15.6%) in the neurogenic claudication group had NP. The LANSS pain score was not significantly correlated with VAS scores for back pain, but did correlate with VAS scores for leg pain (R=0.73, p<0.001) and with ODI back pain scores (R=0.54, p<0.01).
Conclusion
One-third of the patients with LSS had a NP component. The presence of radicular pain correlated strongly with NP. The severity of leg pain and ODI score were also closely related to a NP component. This data may prove useful to understanding the pain characteristics of LSS and in better designing clinical trials for NP treatment in patients with LSS.
The etiology of chronic pain disorders is heterogeneous, comprising nociceptive, neuropathic, and mixed pain pathways. Neuropathic pain (NP) has been defined as pain initiated or caused by a primary lesion or dysfunction of the nervous system1 and which may arise as a consequence of a lesion or disease affecting the somatosensory system. Diabetic neuropathy, post-herpetic neuralgia, trigeminal neuralgia, and post-spinal cord injury pain are classic examples of NP.2 It is believed that NP serves an important role in the pathogenesis of many diseases related to the spine. However, the diagnosis of NP remains clinical and is based on a characteristic symptom profile and diagnostic tests.
Low back pain (LBP) is one of the most challenging chronic pain disorders to treat. Chronic LBP can involve both the back and the legs.3 In addition, both neuropathic and nociceptive pain pathways contribute to lower back and associated leg pain. Generally, the leg pain component is due to NP, and the back pain component is due to nociceptive mechanisms.4 Moreover, about 20% of patients with LBP pain suffer from a NP component.5 It is important to remember that LBP is not a diagnosis but rather describes a constellation of symptoms. LBP is produced by numerous conditions, resulting in difficulty in understanding and anticipating the clinical course. Due to the heterogeneous pathophysiology of LBP, some clinical pain trials have obtained poorer results than other studies of NP conditions, such as diabetic neuropathy.6,7 This highlights a need to better characterize the specific pathophysiology of LBP in order to establish optimal treatment regimens. For example, a medication indicated to treat NP, such as pregabalin, might be considered a first-line drug for patients with lumbar spinal stenosis (LSS) when NP is a significant component of the overall presenting symptom complex.
LSS is a clinical syndrome caused by narrowing of the spinal canal with encroachment on neural structures surrounding bone and soft tissue. Its clinical symptoms vary but appear as a result of neurovascular mechanisms, nerve root excitation, or mechanical compression of the spinal canal. These mechanisms can concur simultaneously. Patients typically present either with LBP and radicular leg pain or with neurogenic claudication. Because lumbar flexion increases the available space in the spinal canal, patients usually complain of clamping pain in the buttocks and legs when walking, which disappears with sitting or lumbar flexion. However, radicular pain, which may not improve with flexion, can also be attributed to spinal stenosis.8,9,10
Spinal stenosis is the most common reason for lumbar spine surgery in middle-age and elderly populations, likely because of the degenerative pathogenesis.11,12 Some studies on the prevalence of NP components in patients with sciatica or radiculopathy have revealed a higher prevalence of NP in patients with severe radiculopathy or neurologic deficits.13,14,15 Attal, et al.14 found that over 30% of patients with chronic LBP had neuropathic limb pain on the DN4 questionnaire. Moreover, over 70% of patients with neurologic deficits had NP. However, few have studied NP pain components in patients with spinal stenosis. In this study, we aimed to determine the extent of the NP component using the Leads Assessment of Neuropathic Symptoms and Signs (LANSS) scale and to identify the relationships between NP and symptom characteristics in patients with LSS scheduled to undergo surgery.
This prospective study was performed between March 2010 and May 2012. Eighty-six consecutive patients with spinal stenosis who were scheduled to undergo spinal surgery were enrolled. All patients had moderate to severe symptoms, such as radicular leg pain and neurogenic claudication, related to LSS. MRI was performed in all patients to obtain confirmatory cross-sectional imaging of the LSS at one or more levels.16,17 Exclusion criteria included cauda equina syndrome, spinal infection, tumor, or spinal fracture. Patients with peripheral arterial occlusive diseases, severe diabetic neuropathy, or diabetic foot were also excluded from this study. All patients who agreed to be involved in this study provided written informed consent. The Institutional Review Board of our hospital approved the study. All patients completed self-assessment questionnaires and provided demographic and clinical information, including socio-demographic data and symptoms related to LSS. Patients were characterized as having predominant radicular pain or neurogenic claudication. Patients with radicular pain that did not improve upon flexion were classified into the radicular pain group.
The intensity of pain in the back and/or legs was measured using a 100-mm visual analog scale (VAS) on which a score of 0 indicates no pain and a score of 100 indicates the worst conceivable pain. Health-related quality of life (HRQoL) was assessed using the Korean version of the Oswestry Disability Index (ODI).18 The LANSS was used to assess the sensory descriptions of pain provided by the patient and from a bedside examination of sensory dysfunction. Positive scores on the LANSS identified patients with pain that was predominantly neuropathic in origin. The sensitivity and specificity were 85% and 80%, respectively.19,20,21 All patients were assessed using the LANSS pain scale. A score <12 indicated that neuropathic mechanisms were unlikely, whereas a score ≥12 suggested that neuropathic mechanisms were likely (Table 1).19 The primary focus of this study was to determine the prevalence of NP components in patients with LSS using the LANSS pain scale. The secondary focus was to explore potential relationships between clinical symptoms and NP.
All qualitative variables are reported as frequencies and percentages. Quantitative variables are described as means with standard deviation. We divided participants into two groups by their main symptoms. Variables were analyzed using Fisher's exact test or the Mann-Whitney U test. The relationships between LANSS score and co-morbidities including diabetes mellitus (DM) and hypertension were assessed using logistic regression analysis. The correlations between LANSS score and continuous variables, such as the VAS and ODI scores, were calculated using Spearman's correlation coefficient. SPSS version 18.0 for Windows (SPSS Inc., Chicago, IL, USA) was used for all statistical analyses. All p-values<0.05 were accepted as statistically significant.
Eighty-six patients (34 men, 52 women; mean age: 66.3±5.9) were included in this study. All patients were diagnosed with LSS using MRI during the preoperative period. The most frequently involved level was L4-5. The mean duration of symptoms was 9 months. The mean intensities of back and leg pain were 4.6±2.3 and 6.2±3.1, respectively. The mean ODI score was 23.4±12.7. The mean LANSS score was 7.2±5.6 (range: 0-24). When organizing patients according to LANSS guidelines,19 31 patients (36.0%) had NP as indicated by scores ≥12 (Table 2).
Seventy-eight percent of patients had both radicular pain and NC. All patients indicated either a predominance of radicular pain over NC or vice versa. There were 41 patients with predominant radicular pain and 45 patients with predominant neurogenic claudication; there were no significant differences in sex, age, BMI, or co-morbidities between these two groups. Forty-one patients were classified as having primary radicular pain symptoms or predominant radicular pain. Grouping primary and predominant NC together, there were 45 patients with neurogenic claudication. VAS scores on the leg were significantly higher in the radicular pain group, while VAS scores for the back were not significantly different between the two groups. Twenty-four patients (63.4%) in the radicular pain group had NP, while only seven patients (15.6%) in the neurogenic claudication group had NP (p<0.05) (Table 3, Fig. 1).
A significant correlation was observed between LANSS and VAS scores for leg pain (R=0.73, p<0.001) and between LANSS and ODI scores (R=0.54, p<0.01). VAS scores for back pain, age, sex, duration of symptoms, and level of involvement did not correlate significantly with LANSS score (Table 4). Also, co-morbidities, including DM or hypertension, were not significantly related with LANSS scores (Table 5).
Lumbar spinal stenosis causes a range of clinical symptoms, including back pain, radicular pain, neurogenic claudication, and neurologic deficits.22 The heterogeneity of the clinical symptoms and pathophysiology of LSS contributes to the difficulty in understanding the actual source of pain. Attal, et al.14 demonstrated a higher prevalence of NP components in patients with chronic LBP. However, that study did not differentiate between the causes of the chronic LBP. In this study, we found a higher prevalence (36%) of NP components in patients with LSS than that reported in other studies of patients with LBP.14,20 In subgroup analysis, patients in the radicular pain group had higher LANSS scores than patients in the claudication group. Almost two-thirds of patients in the radicular pain group had LANSS scores over 12, indicating NP; meanwhile, only seven patients (15.6%) in the claudication group had LANSS scores consistent with NP.
Several screening tools have been introduced to identify NP. The LANSS pain scale was the first tool to be developed and contains five symptom items and two clinical examination items. The LANSS pain scale can distinguish patients with NP from those with nociceptive pain with high reliability and validity.19,20 Adequate psychometric evidence has also been demonstrated for the measurement of treatment effects.20,23
In the present study, a NP component was correlated with leg pain but not back pain. Moreover, radicular pain in patients with LSS seemed to be mainly related to a NP component. Compared to patients in the neurogenic claudication group, more severe leg pain was demonstrated in patients in the radiculopathy group. Kovacs, et al.10 reviewed and reported that patients with radicular pain show poorer outcomes, despite being amenable to surgery. This might be due to the radicular symptoms belonging more to the NP group, with its sensitization of peripheral nerves or the central nervous system, than to the nociceptive pain group. This may represent a mechanism in patients with radicular pain that is more difficult to treat.
LSS can present in several ways but often presents as neurogenic claudication or radicular pain. Neurogenic claudication in patients with LSS is characterized by bilateral or unilateral thigh or calf pain or weakness when walking.24 It is thought that narrowing of the spinal canal results from degenerative changes of the spine, leading to compression or ischemia of the lumbosacral nerve roots.22,25,26 In contrast, radicular pain (e.g., sciatica) is thought to be due to nerve root irritation from chemical or mechanical inflammation or from direct neural compression in the central canal.27,28 Lumbar flexion does not improve radiculopathy, and the symptoms do not always occur independently but rather can be mixed. In our study, patients with LSS also complained of radicular pain and/or neurogenic claudication; however, NP appeared to be more related to radicular pain and not neurogenic claudication. A higher VAS score for leg pain was closely related to higher LANSS score. On correlation analysis, ODI score was also correlated with LANSS score, suggesting that NP results in more leg than back pain and can significantly worsen functional status. As reported by Freynhagen, et al.,4,5,29 leg symptoms are the result of NP, but back pain is not. However, in regard to leg symptoms, radiculopathy was more strongly related to a NP component. Our study suggests that degenerative spinal disorders such as LSS, which produce back and leg pain, have both neuropathic and nociceptive pain components.
Several diseases, such as DM, hypertension, or hyperlipidemia, might involve NP components.30,31 LSS typically presents in aged populations, amongst whom DM and hypertension are very common. In our study population, 70% of participants had hypertension and 56% had DM. Attal, et al.6,7 showed that, among patients with chronic LBP, over 30% have neuropathic limb pain. However, this study did not rule out the effects of DM or hypertension. In contrast, in this study, we excluded patients with severe diabetic neuropathy or peripheral arterial occlusive disease. We also confirmed co-morbidities in patients before surgery and found no differences between the two groups. Given that we excluded the effects of other comorbidities, this study should be more accurate than other studies, perhaps resulting in the much higher percentage of NP in LSS patients.
The treatment of NP is still a challenge. The first step is treatment of the underlying pathology, but the importance of pharmacologic treatment of the NP component is increasing. Various types of drugs, including antidepressants such as selective serotonin norepinephrine reuptake inhibitors (SSNRIs), calcium channel α2-δ ligands, opioid analgesics, and topical lidocaine, have all been recommended.32,33,34,35,36 These drug classes might also be helpful in patients with LSS, especially in cases of severe radiculopathy, since a NP component seems to play an important role in leg pain and the severity of pain. In some randomized clinical trials of patients with spinal stenosis, different results were obtained between patients with neurogenic claudication and patients with radicular pain. Better results from surgery were expected in patients with neurogenic claudication.10,37 This suggests that the characteristics and natural history of pain in patients with LSS are different. It is important to more precisely assess the presenting symptoms of patients with LSS so that each component of pain, including NP, can be targeted with available pharmacologic treatments and in order to better inform patients with varying presenting symptoms about potential surgical outcomes.
Despite methodological problems with this study, including a lack of randomization, the relatively small number of participants, and the inclusion of only patients with planned surgery, it is one of only a few studies that has evaluated the prevalence and characteristics of NP in patients with LSS. This cross-sectional study is limited by a lack of follow-up results. The change of LANSS scale resulting from surgery or conservative treatment, such as drugs, will be highlighted in future steps.
In summary, one-third of patients with LSS had a NP component. Radicular pain correlated more strongly with NP than neurogenic claudication. The severity of leg pain and ODI score also demonstrated strong relationships with a NP component. These results will be useful to understanding the characteristics of pain in LSS and in designing future clinical drug trials.
Figures and Tables
 | Fig. 1Proportion of patients with neuropathic pain by group (neuropathic pain was defined as a LANSS score greater than or equal to 12). LANSS, Leads Assessment of Neuropathic Symptoms and Signs. |
Table 1
Leads Assessment of Neuropathic Symptoms and Signs Scale

Scoring: add values in parentheses for sensory description and examination findings to obtain overall score. Total score (maximum 24). If score<12, neuropathic mechanisms are unlikely to be contribution to the patient's pain. If score≥12, neuropathic mechanisms are likely to be contributing to the patient's pain.
Table 2
Demographic Data
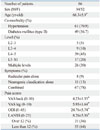
Table 3
Clinical Characteristics of the Patient Groups
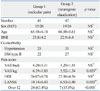
Table 4
Correlation between Pain or Functional Scores and LANSS Scores in Patients with Lumbar Spinal Stenosis (Spearman's Correlation Test)
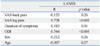
| LANSS | ||
|---|---|---|
| R | p value | |
| VAS back pain | -0.133 | 0.26 |
| VAS leg pain | 0.728 | <0.001 |
| Duration of symptoms | 0.183 | 0.41 |
| ODI | 0.544 | <0.001 |
| Sex | 0.212 | 0.26 |
| Age | -0.097 | 0.37 |
References
1. Merskey H, Bogduk H. Classification of Chronic Pain: descriptions of chronic pain syndromes and definitions of pain terms. 2nd ed. Seattle (DC): IASP Press;1994.
2. Bouhassira D, Lantéri-Minet M, Attal N, Laurent B, Touboul C. Prevalence of chronic pain with neuropathic characteristics in the general population. Pain. 2008; 136:380–387.

3. Deyo RA, Tsui-Wu YJ. Descriptive epidemiology of low-back pain and its related medical care in the United States. Spine (Phila Pa 1976). 1987; 12:264–268.

4. Freynhagen R, Baron R. The evaluation of neuropathic components in low back pain. Curr Pain Headache Rep. 2009; 13:185–190.

5. Freynhagen R, Baron R, Gockel U, Tölle TR. painDETECT: a new screening questionnaire to identify neuropathic components in patients with back pain. Curr Med Res Opin. 2006; 22:1911–1920.

6. Attal N, Cruccu G, Haanpää M, Hansson P, Jensen TS, Nurmikko T, et al. EFNS guidelines on pharmacological treatment of neuropathic pain. Eur J Neurol. 2006; 13:1153–1169.

7. Attal N, Cruccu G, Baron R, Haanpää M, Hansson P, Jensen TS, et al. EFNS guidelines on the pharmacological treatment of neuropathic pain: 2010 revision. Eur J Neurol. 2010; 17:1113–e88.

8. Hall S, Bartleson JD, Onofrio BM, Baker HL Jr, Okazaki H, O'Duffy JD. Lumbar spinal stenosis. Clinical features, diagnostic procedures, and results of surgical treatment in 68 patients. Ann Intern Med. 1985; 103:271–275.
9. Epstein NE, Maldonado VC, Cusick JF. Symptomatic lumbar spinal stenosis. Surg Neurol. 1998; 50:3–10.
10. Kovacs FM, Urrútia G, Alarcón JD. Surgery versus conservative treatment for symptomatic lumbar spinal stenosis: a systematic review of randomized controlled trials. Spine (Phila Pa 1976). 2011; 36:E1335–E1351.
11. Deyo RA, Gray DT, Kreuter W, Mirza S, Martin BI. United States trends in lumbar fusion surgery for degenerative conditions. Spine (Phila Pa 1976). 2005; 30:1441–1445.

12. Weinstein JN, Tosteson TD, Lurie JD, Tosteson AN, Blood E, Hanscom B, et al. Surgical versus nonsurgical therapy for lumbar spinal stenosis. N Engl J Med. 2008; 358:794–810.

13. Rados I, Sakic Zdravcevic K, Hrgovic Z. painDETECT questionnaire and lumbar epidural steroid injection for chronic radiculopathy. Eur Neurol. 2013; 69:27–32.

14. Attal N, Perrot S, Fermanian J, Bouhassira D. The neuropathic components of chronic low back pain: a prospective multicenter study using the DN4 Questionnaire. J Pain. 2011; 12:1080–1087.

15. Mahn F, Hüllemann P, Gockel U, Brosz M, Freynhagen R, Tölle TR, et al. Sensory symptom profiles and co-morbidities in painful radiculopathy. PLoS One. 2011; 6:e18018.

16. Genevay S, Atlas SJ, Katz JN. Variation in eligibility criteria from studies of radiculopathy due to a herniated disc and of neurogenic claudication due to lumbar spinal stenosis: a structured literature review. Spine (Phila Pa 1976). 2010; 35:803–811.

17. Steurer J, Roner S, Gnannt R, Hodler J. LumbSten Research Collaboration. Quantitative radiologic criteria for the diagnosis of lumbar spinal stenosis: a systematic literature review. BMC Musculoskelet Disord. 2011; 12:175.

18. Jeon CH, Kim DJ, Kim SK, Kim DJ, Lee HM, Park HJ. Validation in the cross-cultural adaptation of the Korean version of the Oswestry Disability Index. J Korean Med Sci. 2006; 21:1092–1097.

19. Bennett M. The LANSS Pain Scale: the Leeds assessment of neuropathic symptoms and signs. Pain. 2001; 92:147–157.

20. Bennett MI, Attal N, Backonja MM, Baron R, Bouhassira D, Freynhagen R, et al. Using screening tools to identify neuropathic pain. Pain. 2007; 127:199–203.

21. Kaki AM, El-Yaski AZ, Youseif E. Identifying neuropathic pain among patients with chronic low-back pain: use of the Leeds Assessment of Neuropathic Symptoms and Signs pain scale. Reg Anesth Pain Med. 2005; 30:422–428.

22. Amundsen T, Weber H, Lilleås F, Nordal HJ, Abdelnoor M, Magnaes B. Lumbar spinal stenosis. Clinical and radiologic features. Spine (Phila Pa 1976). 1995; 20:1178–1186.
23. Khedr EM, Kotb H, Kamel NF, Ahmed MA, Sadek R, Rothwell JC. Longlasting antalgic effects of daily sessions of repetitive transcranial magnetic stimulation in central and peripheral neuropathic pain. J Neurol Neurosurg Psychiatry. 2005; 76:833–838.

24. Kalichman L, Cole R, Kim DH, Li L, Suri P, Guermazi A, et al. Spinal stenosis prevalence and association with symptoms: the Framingham Study. Spine J. 2009; 9:545–550.

25. Katz JN, Harris MB. Clinical practice. Lumbar spinal stenosis. N Engl J Med. 2008; 358:818–825.
26. Takahashi K, Kagechika K, Takino T, Matsui T, Miyazaki T, Shima I. Changes in epidural pressure during walking in patients with lumbar spinal stenosis. Spine (Phila Pa 1976). 1995; 20:2746–2749.

27. Kobayashi S, Baba H, Uchida K, Kokubo Y, Kubota C, Yamada S, et al. Effect of mechanical compression on the lumbar nerve root: localization and changes of intraradicular inflammatory cytokines, nitric oxide, and cyclooxygenase. Spine (Phila Pa 1976). 2005; 30:1699–1705.

28. Kobayashi S, Kokubo Y, Uchida K, Yayama T, Takeno K, Negoro K, et al. Effect of lumbar nerve root compression on primary sensory neurons and their central branches: changes in the nociceptive neuropeptides substance P and somatostatin. Spine (Phila Pa 1976). 2005; 30:276–282.

29. Freynhagen R, Baron R, Tölle T, Stemmler E, Gockel U, Stevens M, et al. Screening of neuropathic pain components in patients with chronic back pain associated with nerve root compression: a prospective observational pilot study (MIPORT). Curr Med Res Opin. 2006; 22:529–537.

31. Vranken JH. Mechanisms and treatment of neuropathic pain. Cent Nerv Syst Agents Med Chem. 2009; 9:71–78.

32. Moulin DE, Clark AJ, Gilron I, Ware MA, Watson CP, Sessle BJ, et al. Pharmacological management of chronic neuropathic pain - consensus statement and guidelines from the Canadian Pain Society. Pain Res Manag. 2007; 12:13–21.

33. Gronseth G, Cruccu G, Alksne J, Argoff C, Brainin M, Burchiel K, et al. Practice parameter: the diagnostic evaluation and treatment of trigeminal neuralgia (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology and the European Federation of Neurological Societies. Neurology. 2008; 71:1183–1190.

34. Jensen TS, Madsen CS, Finnerup NB. Pharmacology and treatment of neuropathic pains. Curr Opin Neurol. 2009; 22:467–474.

35. Baron R, Freynhagen R, Tölle TR, Cloutier C, Leon T, Murphy TK, et al. The efficacy and safety of pregabalin in the treatment of neuropathic pain associated with chronic lumbosacral radiculopathy. Pain. 2010; 150:420–427.

36. Baron R, Binder A, Wasner G. Neuropathic pain: diagnosis, pathophysiological mechanisms, and treatment. Lancet Neurol. 2010; 9:807–819.

37. Ammendolia C, Stuber K, de Bruin LK, Furlan AD, Kennedy CA, Rampersaud YR, et al. Nonoperative treatment of lumbar spinal stenosis with neurogenic claudication: a systematic review. Spine (Phila Pa 1976). 2012; 37:E609–E616.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download