Abstract
Purpose
The aim of this study was to analyze the results of children treated with hemodialysis (HD) at Severance Hospital over 35 years in terms of incidence, etiologies, characteristics, complications, and clinical outcomes.
Materials and Methods
We analyzed 46 children admitted to Severance Hospital who had undergone HD between January 1979 and December 2013.
Results
The main etiologies of the 23 end-stage renal disease (ESRD) patients who had received HD were chronic glomerulonephritis (7 patients, 30.4%) and congenital anomalies of the kidney and urinary tract (7 patients, 30.4%), whereas the etiology of the 23 acute kidney injury (AKI) patients was hemolytic uremic syndrome (6 patients, 26.1%). Compared with ESRD patients, hemocatheter placement in the femoral vein was preferred over the subclavian or internal jugular vein in the AKI patients (p=0.012). The most common complication was catheter related complication (10 patients, 21.7%). The site of hemocatheter insertion was not related to the frequency of oozing. Placing the hemocatheter in the femoral vein resulted in significantly more events of catheter obstruction than insertion in the internal jugular vein or the subclavian vein (p=0.001). Disequilibrium syndrome occurred more frequently in older patients (p=0.004), as well as patients with a greater body weight (p=0.008) and a higher systolic and diastolic blood pressure before HD (systolic: p=0.021; diastolic: p=0.040).
End-stage renal disease (ESRD) is the terminal stage of chronic kidney disease (CKD), and renal replacement therapy (RRT) is necessary to sustain life among ESRD patients who are awaiting kidney transplantation (KT).1 Acute kidney injury (AKI) is a rapid and reversible deprivation of kidney function, leading to retention of fluid and nitrogenous wastes and derangement of acid-base balance and electrolytes.2 As the prevalence of ESRD and AKI patients requiring RRT has recently increased, access to various renal replacement modalities such as peritoneal dialysis (PD), hemodialysis (HD), and continuous hemodiafiltration has improved accessibility for the treatment of pediatric patients with ESRD and AKI.1
Among dialysis modalities, PD is preferred in young children with ESRD due to better hemodynamic stability and more preservation of residual renal function.1,3 However, HD is also used as a short-term bridge to renal transplantation or in situations when PD cannot be carried out for various reasons.4,5,6 In children with AKI, the application of continuous hemodiafiltration is recently increasing, as strict volume control and better nutritional support are possible.7 However, given that continuous hemodiafiltration is usually performed in the intensive care unit (ICU), HD can be applied in less severe AKI children, who do not need to admitted to the ICU.
HD has been used in pediatric patients since the 1950s.8 Over the past decade, the proportion of pediatric patients receiving HD as RRT has gradually increased as equipment and techniques for HD have improved, even for small children.9 However, there have been very few data on HD in pediatric patients despite the increasing frequency of HD.10 The first usage of HD on a pediatric patient in Korea was performed at our center in 1979 on a 13-year-old boy with ESRD caused by vesicoureteral reflux, who was treated for one month before renal transplantation.11 For the continued development of HD as an optimal renal replacement therapeutic modality in pediatric patients, accurate data are required based on long-term experience.3
The purpose of our study was to review the data of pediatric patients treated with HD at Severance Hospital over the last 35 years in terms of incidence, etiologies, characteristics, complications, and clinical outcomes.
Data from all patients who had received HD at Severance Hospital in South Korea from January 1979 to December 2013 were retrospectively reviewed. Ages of all patients were 18 years or less at the commencement of HD. We identified 54 children admitted to Severance Hospital who had undergone HD during the study period. Among them, 46 patients with available medical records were retrospectively reviewed. These 46 patients were divided into two groups according to reasons for receiving HD: AKI and ESRD. Patients were followed until death, renal transplantation, withdrawal from dialysis, or recovery of renal function.
The following information was collected for all patients: age at the commencement of HD, body weight, body weight change after HD, gender, frequency of HD sessions, duration of the first HD session, reason for receiving HD, reason for selecting HD as a RRT modality, site of initial vascular access, complications during HD, symptoms during HD, kinds of anti-coagulants during HD, prognosis of the patients, and laboratory investigations [complete blood count, initial (pre-HD) blood urea nitrogen (BUN), post-HD BUN, reduction ratio of BUN after HD, electrolytes before and after the first HD session, and blood pressure before and after the first HD session]. We calculated the BUN reduction ratio with the BUN before and after HD in patients according to the following equation: BUN reduction ratio=100×(pre-HD BUN-post-HD BUN/pre-HD BUN). HD was prescribed three to four times per week and lasted 2-4 hours per session. The blood flow rate (BFR) is usually described as "mL/min" in adults; however, as our patients included both small and large children, we described it as "mL/kg/min" for accurate analysis. The model of the infant dialysis machine was not available before 2005. After the year 2005, HD was performed with Fresenius Medical Care 4008-S machines and Helixone dialysis filters (Fx paed®, Fresenius, Bad Homburg, Germany).
Results were evaluated using frequencies and percentages for categorical variables and means with standard deviation or median with ranges for continuous variables. Data were analyzed with a t-test, a chi-square test, Fisher's exact test, or the Mann-Whitney U test, using SPSS software (version 20.0, SPSS Inc., Chicago, IL, USA) for Windows. A p-value<0.05 was considered statistically significant.
From January 1979 to December 2013 a total of 54 patients who were younger than 18 years were found to have been treated with HD. Among these patients, 46 (29 male, 63.0%; 17 female, 37.0%) had sufficient data for analysis in this study. The number of patients receiving HD increased significantly after the year 2000, which was dependent on the increasing application of HD due to improving technology (Fig. 1).
Patients were divided into two groups depending on the reason for HD: ESRD (23 patients, 50%) and AKI (23 patients, 50%). The main etiologies for HD among ESRD patients were chronic glomerulonephritis [7 patients, 30.4%: focal segmental glomerulosclerosis (4 patients), lupus nephritis (1 patient), IgA nephritis (1 patient), and IgM nephritis (1 patient)] and congenital anomalies of the kidney and urinary tract (CAKUT) [7 patients, 30.4%: vesicoureteral reflux (3 patients), dysplastic or hypoplastic kidney (3 patients), and polycystic kidney (1 patient)]. On the other hand, the most common cause of AKI was hemolytic uremic syndrome (6 patients, 26.1%). Other etiologies of each group are described in Table 1.
The average frequency of whole HD sessions per each patient was 213.4±74.9 sessions (range 2-997 sessions, median 13.0 sessions) in ESRD patients and 2.2±0.6 sessions (range 1-30 sessions) in AKI patients. The mean duration of the first HD session in the ESRD patient group was 2.1±0.9 hours (range 2-4 hours, median 2.0 hours) and 2.2±0.6 hours (range 1.5-4 hours, median 2.3 hours) in the AKI group. We determined that the duration of the first dialysis session was relatively shorter than the duration of maintenance HD in order to prevent the occurrence of disequilibrium syndrome and to allow patients to adapt to solute removal and ultrafiltration. The mean age at the time of HD initiation was 10.1±4.6 years old (range 1.1-18.0) in ESRD patients and 8.4±5.2 years old (range 1.7-18.0) in AKI patients. There was a statistically significant difference of onset age between AKI and ESRD patients (p=0.002). Grouping by age distribution, the age group including patients 5 to 9 years old was most dominant among ESRD patients (9 patients, 39.1%), and those 0 to 4 years old were the largest age group among AKI patients (10 patients, 43.4%). When onset age was divided into groups, there was no statistical difference between AKI and ESRD patients (p=0.073).
The average weight of patients at the time of HD therapy was 24.5±16.1 kg (range 9.4-52.3 kg) in the ESRD patient group and 31.3±19.4 kg (range 11.8-71.0 kg) in AKI patients. There was no statistical difference in body weight between the two groups (p=0.391) (Table 1). The BFR depends on the body weight and blood pressure of the patient, as vital signs of small and hypotensive children could change easily according to relatively small changes of body volume upon initial HD; additionally, small children have small-size hemocatheters, which cannot endure a high BFR. In our report, the mean BFR was 5.1±2.4 mL/kg/min (3.0-10.7 mL/kg/min) (Table 2).
Hemocatheters were inserted for vascular access for all patients. The most commonly used site for the hemocatheter was the internal jugular vein (24 patients, 52.2%), followed by the subclavian vein (13 patients, 28.3%) and the femoral vein (9 patients, 19.5%). In contrast to ESRD patients, hemocatheter placement in the femoral vein was preferred over the subclavian or internal jugular vein in AKI patients (p=0.012). Among the ESRD patients, a hemocatheter was changed to an arteriovenous fistula (AVF) in 3 patients and to a permanent catheter in 14 patients for maintenance of HD. The permanent catheter was flushed with heparin (1000 IU) to maintain patency between each HD session. AVF formations were performed in 3 children. One patient needed long-term HD due to rejection after KT. Two boys needed long-term HD until they found suitable kidney donors, as they did not have available kidney donors in their family. In the first HD session, heparin was used for 17 patients (37.0%), and nafamostat was administrated to 1 patient (2.2%), both as anti-coagulation drugs for the maintenance of the catheter. One patient with nafamostat in the ESRD group showed disseminated intravascular coagulation. Every patient used heparin at the end of whole sessions of HD. Except for 8 patients who had bleeding tendencies, administrations of anti-coagulants at the first session of HD were followed by the preference of nephrologists.
The most common type of complication of HD among all 46 patients was a catheter-related complication (10 patients, 21.7%) followed by disequilibrium syndrome (9 patients, 20.0%). The remaining 27 patients had no significant complications, such as disequilibrium syndrome, catheter-related complications, electrolyte imbalance, cardiac problems, or hypotension.
The most frequent catheter-related complication during HD was oozing (4 patients, 8.7%), followed by obstruction (3 patients, 6.5%) and line extrusion (2 patients, 4.3%). Only one patient (2.2%) had a catheter-related infection. The site of hemocatheter insertion was not significantly related to oozing; however, a hemocatheter placed in the femoral vein had significantly more events of catheter obstruction than those inserted in the internal jugular vein or the subclavian vein (p=0.001).
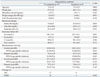
At our center, 9 patients presented symptoms of disequilibrium syndrome. This complication occurred more frequently in older patients (no symptoms: 8.2±5.0 years; symptoms: 12.2±2.9 years; p=0.004), patients with a greater body weight (no symptoms: 27.2±2.8 kg; symptoms: 44.1±15.3 kg; p=0.008), and those with a greater change in body weight after HD (no symptoms: 0.5±0.3 kg; symptoms: 1.4±0.1 kg; p=0.037). However, there were few data for body weight after HD (13 patients with no symptoms and 4 patients with symptoms). Furthermore, patients with a higher systolic blood pressure (no symptoms: 123.0±15.6 mm Hg; symptoms: 140.1±21.6 mm Hg; p=0.021) and diastolic blood pressure (no symptoms: 74.3±13.9 mm Hg; symptoms: 85.7±16.3 mm Hg; p=0.040) before HD more commonly showed disequilibrium syndrome. In addition, as the definition of hypertension (HTN) is different for all ages and genders in children and adolescents, we attempted to categorize the blood pressures (BPs) of our patients according to the Fourth Report of National High Blood Pressure Education Program Working Group on High Blood Pressure.12 Additionally, we studied correlations between the age-specific HTN and frequency of disequilibrium syndrome occurrences. We found that patients with age-specific higher systolic and diastolic BPs before HD more commonly presented disequilibrium syndrome, with the results showing statistical significance (p=0.013). However, there was no statistical significance in a multivariate analysis comparing age, weight, BFR, systolic blood pressure before HD, and diastolic blood pressure before HD with the occurrence of disequilibrium syndrome (Table 3).
Among the 23 patients with ESRD, 11 patients received KT and 11 were switched to PD for maintenance of RRT. The average periods of dialysis were 61.8±99.0 days (median 58 days) before KT and 36.7±25.7 days (median 30 days) before conversion to PD.
Among 23 patients with AKI, 20 patients recovered from AKI status (30.4±29.1 days, median 24 days), and 1 patient who progressed to ESRD switched from HD combined with continuous hemodiafiltration to PD (HD duration: 3 days). Three children (6.5%) expired during the observation period (2 AKI patients and 1 ESRD patient). The causes of death were septic shock in 1 AKI patient (expired 7 days after starting HD), aggravating disseminated intravascular coagulation in 1 AKI patient (expired 25 days after starting HD), and uremic cardiomyopathy in 1 ESRD patient (expired 5 years after starting HD).
The outlook for pediatric patients with RRT has notably improved over the last 20 years.13 As a consequence, the number of pediatric HD patients has increased since the first pediatric HD at our center in 1979.11 In fact, the proportion of those younger than 5 years old has particularly increased since the year 2000. In addition, the mortality of pediatric ESRD patients on HD was very low: only one patient (4.3%) died during the HD treatment period at our center. According to reports from other countries, the mortality rate of pediatric ESRD patients on HD is 4.2-26.7%,14,15,16 and the main cause of mortality in pediatric patients is cardiovascular disease.14,17 In our center, we observed only one patient who died due to uremic cardiomyopathy.
CAKUT is the main etiology of CKD (34-43%) among pediatric ESRD patients in developed countries, such as Europe, Japan, Australia, New Zealand, and the United States,18,19,20 whereas chronic glomerulonephritis is the main cause of CKD in various other countries and regions such as India, Latin America, Southeast Asia, and sub-Saharan Africa (30-60%).21,22,23,24 In our center, glomerulonephritis and CAKUT occurred with the same frequency (30.4% each) as the leading causes of CKD.
The most common etiology of AKI at our center was hemolytic uremic syndrome (6 patients, 26.1%) and glomerulonephritis (4 patients, 17.4%). Regarding the etiologies of AKI, our center showed a changing trend from hemolytic uremic syndrome and glomerulonephritis in the 1980s to acute tubulonephritis, congenital heart disease, and nephrotoxic medications in the 2010s, similar to other studies.1,25
At our hospital, a double-lumen, un-cuffed hemocatheter was the first choice for children on short-term and long-term HD. The rate of catheter-related complications was very low at our center. Such remarkable results could be attributed to the performance of skillful doctors with radiologic devices such as ultrasonography and C-arm, as well as systematic aseptic catheter management. Moreover, the insertion site of the hemocatheter had no impact on the frequency of catheter-related oozing in our study. However, hemocatheters placed in the femoral vein had significantly more obstruction events than those placed in the internal jugular vein or the subclavian vein.
Current guidelines from Europe and the United States on vascular access for long-term HD suggest that an AVF should be considered in pediatric patients rather than a hemocatheter, whereas temporary hemocatheters are the first choice for children requiring short-term HD.26,27 In our center, only 3 patients had an AVF for long-term HD. The reason for this low number may be due to relatively prompt KT from parents, and as a result, there were very few pediatric patients who needed a long term maintenance HD, which would normally require an AVF formation.
Dialysis disequilibrium syndrome was one of the rare complications associated with HD. This syndrome is characterized by neurologic deteriorations such as headache, numbness, restlessness, seizure, or mental status changes.28,29 The causes and risk factors of this syndrome are still not exactly known.28,29
Despite the development of HD over the years, the etiologies and risk factors of this syndrome remain poorly defined, especially in children. Some reports have suggested that the risk factors for disequilibrium syndrome were a markedly high BUN, a high BUN reduction ratio, CKD rather than AKI, metabolic acidosis, pediatric patients, electrolyte imbalances (especially hyponatremia), or pre-existing central nervous system lesions such as malignant HTN before HD.28,29,30,31 However, studies on disequilibrium syndrome in pediatric patients undergoing HD have not yet been published. According to our study, older and heavier patients had more frequencies of occurring disequilibrium syndrome during HD. Furthermore, patients with high systolic and diastolic blood pressure before HD also had a greater frequency of disequilibrium syndrome during HD. Along these lines, Arieff, et al.30,32 reported that patients with conditions such as malignant HTN develop cerebral edema easier than patients with normal blood pressure, such that these patients had a greater tendency to develop disequilibrium syndrome during HD. Although our data shows that a greater change in body weight after HD can increase the risk of disequilibrium syndrome, the small number of data collected is a limitation of this study. In addition, patients with a fast BFR (described as "mL/min") upon HD had a higher frequency of disequilibrium syndrome in our study. This result was consistent with the report from Bagshaw, et al.31 and Flannery, et al.33 that reducing the blood flow rate during HD lowered the rate of disequilibrium syndrome. However, when we described BFR as including body weight (mL/kg/min), it did not correlate with the frequency of disequilibrium syndrome. Future large-scale, long-term studies will be required to elucidate the pathophysiology behind this phenomenon.
Lastly, the BUN reduction ratio, BUN levels before and after HD, and the use of mannitol were not statistically related to the occurrence of disequilibrium syndrome in our study. Our center prescribed the first HD to patients as a goal of reducing BUN concentration by less than 40% in order to prevent disequilibrium syndrome, as some reports about disequilibrium syndrome had indicated.28,29 As a result of our analysis, we could not conclude that a high BUN reduction ratio was a risk factor in disequilibrium syndrome.
There are some limitations in our study. First, there were limitations of the data analysis due to insufficient old data and missing data of patients under 1 year old. However, our study is the first to describe the etiologies, characteristics, complications, and clinical outcomes of pediatric patients on HD in Korea and also the first in the world to analyze the risk factors of the development of disequilibrium syndrome in pediatric patients on HD. Our results demonstrate that HD can be sufficiently carried out, even in infants. The number of pediatric patients with AKI and ESRD receiving HD has recently increased, and outcomes have improved significantly. Based on the experience of using HD for children at our center, we believe that HD should be more available to children as a safe modality for RRT. Given that the application of HD to small children is not always possible in tertiary hospitals in Korea, the transfer of patients who require HD to available centers may also be necessary.
In conclusion, according to our study, we conclude that HD an effective renal replacement modality for pediatric patients with AKI and ESRD. Further advancements in HD techniques will improve the outcomes of HD in pediatric patients.
Figures and Tables
Fig. 1
Incidence of acute kidney injury and end-stage renal disease for patients who received HD during a 35-year period at Severance Hospital. AKI, acute kidney injury; ESRD, end-stage renal disease; HD, hemodialysis.

Table 1
Clinical Characteristics of 46 Patients on Hemodialysis
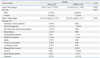
Table 2
Laboratory Characteristics and Blood Pressure of 46 Patients on Hemodialysis
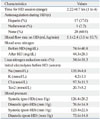
Table 3
Risk Factors for Disequilibrium Syndrome
References
1. Sethi SK, Bunchman T, Raina R, Kher V. Unique considerations in renal replacement therapy in children: core curriculum 2014. Am J Kidney Dis. 2014; 63:329–345.

2. Goldstein SL. Overview of pediatric renal replacement therapy in acute kidney injury. Semin Dial. 2009; 22:180–184.

3. Goldstein SL. Hemodialysis in the pediatric patient: state of the art. Adv Ren Replace Ther. 2001; 8:173–179.

4. Walters S, Porter C, Brophy PD. Dialysis and pediatric acute kidney injury: choice of renal support modality. Pediatr Nephrol. 2009; 24:37–48.

5. Harshman LA, Neuberger ML, Brophy PD. Chronic hemodialysis in pediatric patients: technical and practical aspects of use. Minerva Pediatr. 2012; 64:159–169.
6. Paganini EP, Vidt DG. Renal replacement therapy utilizing hemodialysis and peritoneal dialysis. Urol Clin North Am. 1983; 10:347–367.

7. Sutherland SM, Alexander SR. Continuous renal replacement therapy in children. Pediatr Nephrol. 2012; 27:2007–2016.

8. Mateer FM, Greenman L, Danowski TS. Hemodialysis of the uremic child. AMA Am J Dis Child. 1955; 89:645–655.

9. Warady BA, Neu AM, Schaefer F. Optimal care of the infant, child, and adolescent on dialysis: 2014 update. Am J Kidney Dis. 2014; 64:128–142.

10. Wedekin M, Ehrich JH, Offner G, Pape L. Renal replacement therapy in infants with chronic renal failure in the first year of life. Clin J Am Soc Nephrol. 2010; 5:18–23.

11. Kim PK, Lee C, Lee JS, Yun DJ, Park KL, Kwon TJ, et al. The first case of renal transplatation in childhood in Korea. J Korean Pediatr Soc. 1980; 23:674–681.
12. National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents. The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics. 2004; 114:2 Suppl 4th Report. 555–576.
13. Fischbach M, Edefonti A, Schröder C, Watson A. European Pediatric Dialysis Working Group. Hemodialysis in children: general practical guidelines. Pediatr Nephrol. 2005; 20:1054–1066.

14. Youssef DM, Neemat-Allah MA. Hemodialysis in children: eleven years in a single center in Egypt. Iran J Kidney Dis. 2013; 7:468–474.
15. Abdelraheem M, Ali el-T, Osman R, Ellidir R, Bushara A, Hussein R, et al. Outcome of acute kidney injury in Sudanese children - an experience from a sub-Saharan African unit. Perit Dial Int. 2014; 34:526–533.

16. Jiang Y, Shen Y, Lau KK. Survey of chronic haemodialysis in children between 2007 and 2012 in China. Nephrology (Carlton). 2014; 19:375–378.

17. Chavers BM, Li S, Collins AJ, Herzog CA. Cardiovascular disease in pediatric chronic dialysis patients. Kidney Int. 2002; 62:648–653.

18. Orr NI, McDonald SP, McTaggart S, Henning P, Craig JC. Frequency, etiology and treatment of childhood end-stage kidney disease in Australia and New Zealand. Pediatr Nephrol. 2009; 24:1719–1726.

19. Lewis MA, Shaw J, Sinha MD, Adalat S, Hussain F, Castledine C, et al. UK Renal Registry 12th Annual Report (December 2009): chapter 14: demography of the UK paediatric renal replacement therapy population in 2008. Nephron Clin Pract. 2010; 115:Suppl 1. c279–c288.
20. Hattori S, Yosioka K, Honda M, Ito H. Japanese Society for Pediatric Nephrology. The 1998 report of the Japanese National Registry data on pediatric end-stage renal disease patients. Pediatr Nephrol. 2002; 17:456–461.

21. Gulati S, Mittal S, Sharma RK, Gupta A. Etiology and outcome of chronic renal failure in Indian children. Pediatr Nephrol. 1999; 13:594–596.

22. Mong Hiep TT, Janssen F, Ismaili K, Khai Minh D, Vuong Kiet D, Robert A. Etiology and outcome of chronic renal failure in hospitalized children in Ho Chi Minh City, Vietnam. Pediatr Nephrol. 2008; 23:965–970.

23. Orta-Sibu N, Lopez M, Moriyon JC, Chavez JB. Renal diseases in children in Venezuela, South America. Pediatr Nephrol. 2002; 17:566–569.

24. Harambat J, van Stralen KJ, Kim JJ, Tizard EJ. Epidemiology of chronic kidney disease in children. Pediatr Nephrol. 2012; 27:363–373.

25. Hui-Stickle S, Brewer ED, Goldstein SL. Pediatric ARF epidemiology at a tertiary care center from 1999 to 2001. Am J Kidney Dis. 2005; 45:96–101.

26. Wartman SM, Rosen D, Woo K, Gradman WS, Weaver FA, Rowe V. Outcomes with arteriovenous fistulas in a pediatric population. J Vasc Surg. 2014; 60:170–174.

27. Chand DH, Valentini RP. International pediatric fistula first initiative: a call to action. Am J Kidney Dis. 2008; 51:1016–1024.

28. Zepeda-Orozco D, Quigley R. Dialysis disequilibrium syndrome. Pediatr Nephrol. 2012; 27:2205–2211.

29. Patel N, Dalal P, Panesar M. Dialysis disequilibrium syndrome: a narrative review. Semin Dial. 2008; 21:493–498.
30. Arieff AI. Dialysis disequilibrium syndrome: current concepts on pathogenesis and prevention. Kidney Int. 1994; 45:629–635.

31. Bagshaw SM, Peets AD, Hameed M, Boiteau PJ, Laupland KB, Doig CJ. Dialysis Disequilibrium Syndrome: brain death following hemodialysis for metabolic acidosis and acute renal failure--a case report. BMC Nephrol. 2004; 5:9.
32. Arieff AI, Lazarowitz VC, Guisado R. Experimental dialysis dis equilibrium syndrome: prevention with glycerol. Kidney Int. 1978; 14:270–278.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download