Abstract
Purpose
Fluoroquinolones, rapidly gaining prominence in treatment of Stenotrophomonas maltophilia (SMP), are noted for their potency and tolerability. However, SMP may rapidly acquire resistance to fluoroquinolones. We evaluated associations of clinical factors with acquisition of levofloxacin resistance (LFr) in SMP.
Materials and Methods
Our retrospective cohort study was based on patient data collected between January 2008 and June 2010. Through screening of 1275 patients, we identified 122 patients with data for SMP antibiotic susceptibility testing in ≥3 serial SMP isolates.
Results
We assigned the 122 patients to either the SS group (n=54) in which levofloxacin susceptibility was maintained or the SR group (n=31) in which susceptible SMP acquired resistance. In multivariate regression analysis, exposure to levofloxacin for more than 3 weeks [odds ratio (OR) 15.39, 95% confidential interval (CI) 3.08-76.93, p=0.001] and co-infection or co-colonization with Klebsiella pneumoniae resistant to levofloxacin (OR 4.85, 95% CI 1.16-20.24, p=0.030) were independently associated with LFr acquisition in SMP.
Stenotrophomonas maltophilia (S. maltophilia) is an emergent pathogen in healthcare facilities worldwide, causing mainly pneumonia or septicemia related or unrelated to catheter use. Patients with immunodeficiency are especially at risk.1,2,3 Serious infection attributed to S. maltophilia is a significant cause of mortality in patients with hematologic malignancies,4 with mortality rates up to 37.5%.5
S. maltophilia is intrinsically resistant to antibiotics that could be excluded by multidrug efflux pumps and/or inactivated by β-lactamase and aminoglycoside-modifying enzymes.6 Trimethoprim-sulfamethoxazole (TMP-SMX), historically the first line of defense in S. maltophilia infection,7 may induce resistance related to class 1 integrons containing the sul1 sulfonamide resistance gene and insertion element common region elements containing the sul2 resistance gene.6,8,9 However, rates reported for resistance to TMP-SMX in S. maltophilia are generally less than 10%.10,11,12
Clinically, the use of TMP-SMX in S. maltophilia infection is limited by adverse effects of the drug, including skin eruptions, hepato- and renal toxicity, and bone marrow suppression.7,13 Additionally, resistance to TMP-SMX in S. maltophilia is increasing at certain centers.8,13
Fluoroquinolones, including levofloxacin and moxifloxacin, are an attractive alternative for treating S. maltophilia infection, because they are well-tolerated and effective, compared to TMP-SMX, and because of their low rates of microbial resistance.7,11,13,14,15,16 However, S. maltophilia can rapidly acquire resistance to fluoroquinolones, especially in monotherapy, and this may limit their use in combination therapy.17 In this study, we investigated the poorly understood relationships between clinical factors and the acquisition of levofloxacin resistance in S. maltophilia.
This retrospective cohort study was conducted at Severance Hospital, a 2000-bed university-affiliated teaching hospital and tertiary care referral center in Seoul, South Korea, based on data collected from January 2008 to June 2010. Patients who met all of the following conditions were eligible for this study: 1) age 18 years old or older; 2) ≥3 serial isolations of S. maltophilia by culture, accompanied by in vitro antimicrobial susceptibility testing (AST) in any clinical specimen; and 3) results for three or more consecutive ASTs of S. maltophilia for levofloxacin in clinical specimens from the same system (e.g., the respiratory, urinary or biliary tract, peritoneal or pleural fluid, or an external wound) at intervals from 3 days to 3 months.
We screened the data for 3029 S. maltophilia isolates from 1275 patients, and identified 528 isolates with data for serial isolation and all AST results from 122 patients (Fig. 1). We stratified these 122 patients into either the SS group (n=54) whose records showed maintenance of levofloxacin susceptibility from the first S. maltophilia isolate to the last isolate recorded or the SR group (n=31) with data confirming transition from levofloxacin susceptibility to resistance in serial S. maltophilia isolates. However, we excluded an RR group (85 isolates from 21 patients) whose records revealed the maintenance of levofloxacin resistance from the first S. maltophilia isolate to the last isolate recorded from our study analysis. Additionally, patients (12 isolates from 4 patients) with data confirming transition from levofloxacin resistance to susceptibility and patients (86 isolates from 12 patients) with inconstant AST results for levofloxacin were excluded from the analysis (Fig. 1).
All bacterial species were identified using conventional methods and/or the ATB 32GN system (bioMerieux, Marcy l'Etoile, France). Antimicrobial susceptibility was tested by the Clinical and Laboratory Standards Institute (CLSI) agar dilution method.18 The antimicrobial agents used for AST were TMP-SMX (Dong Wha Pharmaceutical Co. Ltd., Seoul, Korea); levofloxacin (Daiichi Sankyo Co. Ltd., Tokyo, Japan); minocycline (SK Chemicals Co. Ltd., Life Sciences, Seoul, Korea); ceftazidime, amikacin, and gentamicin (Sigma Chemical Co., St. Louis, MO, USA); tigecycline (Wyeth Research, Pearl River, NY, USA); imipenem (Choongwae Pharma Corp., Seoul, Korea); and piperacillin/tazobactam (Yuhan Co. Ltd., Seoul, Korea).
The index isolate was defined as the last levofloxacin-susceptible isolate cultured from a patient in the SS group and as the first levofloxacin-resistant isolate from one patient in the SR group.
The clinical data at the time of identification of the index isolate were collected through review of electronic medical records. Coexisting conditions of interest included length of hospital stay, intensive care unit admission, use of mechanical ventilation, current tracheostomy status, acute renal failure (ARF) with renal replacement therapy (RRT), neutropenia, and steroid or immunosuppressant use, as well as the Charlson's comorbidity index score. We recorded the history and total duration of all systemic antibiotic exposures within 3 months from identification of the index isolate. To evaluate the effect of co-infection or co-colonization with other bacteria on the acquisition of resistance to levofloxacin in S. maltophilia, the data for co-infection or co-colonization were evaluated in parallel with results of AST for fluoroquinolones.
Categorical data are expressed as a number (percent). If the continuous variables had normal distribution, these were expressed as mean±standard deviation. Continuous variables without normal distribution were expressed as median (interquartile range). We used Student's t-test or Mann-Whitney U test and the χ2 test or Fisher's exact test to compare continuous and categorical variables, respectively, in a univariate analysis of clinical characteristics in the SR and SS groups. Variables with p-values less than 0.10 in univariate analysis were included in a multivariate logistic regression analysis to identify clinical factors associated with acquisition of levofloxacin resistance in S. maltophilia. Results from the multivariate analysis are expressed as an odds ratio (OR) and 95% confidence intervals (CIs). A two-tailed p-values <0.05 was taken to indicate significance. All statistical analyses were performed using SPSS 18.0 (SPSS Inc., Chicago, IL, USA).
The SS and SR groups were similar in age and gender (Table 1). A significantly higher proportion of patients in the SR group than in the SS group were in ARF requiring RRT (25.8% vs. 7.4%, p=0.026). Other coexisting conditions did not differ significantly between the two groups (Table 1).
The total duration of exposure to levofloxacin within 3 months from identification of the index isolate was significantly longer in the SR group [33 (9-42) days] than the SS group [11 (4-64) days, p=0.044] (Table 2). However, the total duration of exposure to anti-pseudomonal penicillins, 3rd cephalosporins, 4th cephalosporins, ciprofloxacin, carbapenems, aminoglycosides, glycopeptides, and metronidazole did not show significant differences between the SS and SR groups (Table 2). Co-infection or co-colonization with ciprofloxacin-resistant Staphylococcus aureus was significantly more common in the SS group than in the SR group (31.5% vs. 9.7%, p=0.032). The SR group had significantly higher percentages of co-infection or co-colonization with any gram-negative bacteria and with Klebsiella pneumonia resistant to levofloxacin (58.1% vs. 35.2%, p=0.041; 32.3% vs. 9.3%, p=0.007, respectively) (Table 3).
In the final multivariate logistic regression analysis, exposure to levofloxacin for more than 3 weeks (OR 15.39, 95% CI 3.08-76.93, p=0.001) and co-infection or co-colonization with K. pneumoniae resistant to levofloxacin (OR 4.85, 95% CI 1.16-20.24, p=0.030) were clinical factors independently associated with the acquisition of resistance to levofloxacin in S. maltophilia (Table 4).
This study confirmed the acquisition of levofloxacin resistance in S. maltophilia during clinical application of the drug. S. maltophilia may potentially develop resistance to fluoroquinolones through various mechanisms, including 1) efflux pump systems, such as SmeABC and SmeDEF,19,20 or 2) mutations in outer-membrane porin proteins.21 A previous study suggested that spontaneous mutations conferring resistance to fluoroquinolones occur at frequencies between 10-5 to 10-752 after quinolones therapy in S. maltophilia.22 In a clinical study at the MD. Anderson Cancer Center, use of fluoroquinolones in the previous 90 days was independently associated with emergence of multidrug resistant S. maltophilia infection in cancer patients.23 In a case-control study, Spanik, et al.24 found a positive association of prophylactic fluoroquinolones use with bacteremia by MDR gram-negative bacilli including S. maltophilia in neutropenic cancer patients. Thus experimental and clinical data from multiple studies, including this one, associate fluoroquinolones exposure with acquired resistance to fluoroquinolones in S. maltophilia.
Co-infection or co-colonization with levofloxacin-resistant K. pneumoniae presented another independent clinical factor in acquisition of levofloxacin resistance in S. maltophilia. Co-infection and co-colonization with other bacterial species, especially those with resistance to fluoroquinolones, are clinically relevant because the species may actively exchange drug resistance genes by means of plasmids and transposons.25 Clinical co-infection of S. maltophilia with other pathogens (e.g., other glucose-non-fermenting gram-negative bacteria including Pseudomonas aeruginosa, Burkholderia species and Acinetobacter baumannii; Enterobacteriaceae including Escherichia coli, Klebsiella species and Enterobacter species; Staphylococcus aureus including methicillin-resistant S. aureus; Enterococcus species; Bacteroides species; and Candida albicans) is confirmed through recovery of multiple species from patient samples.26,27,28,29 S. maltophilia can also exchange antibiotic-resistance genes with gram-positive and other gram-negative bacteria.30 The Smqnr gene present in the bacterial chromosome contributes to intrinsic quinolones resistance in S. maltophilia.31 Although S. maltophilia isolates harboring Smqnr genes could serve as a reservoir for horizontal transfer of these genes into Enterobacteriaceae, this event is unlikely to occur because plasmids containing Smqnr are unstable.21,32 As the possibility and mechanism for the transfer of levofloxacin resistance gene from other gram-negative bacteria to S. maltophilia have not been fully evaluated, further in vitro study is warranted to verify the association of S. maltophilia with K. pneumoniae with levofloxacin resistance.
To our knowledge, no study has systematically evaluated clinical factors that influence acquisition of levofloxacin resistance in S. maltophilia. To identify such factors, we followed levofloxacin susceptibility in serial isolates of S. maltophilia from 122 individual patients (i.e., longitudinally) and compared characteristics of patient subgroups in which susceptibility was maintained (the SS group) and not maintained (the SR group). Although patients with cystic fibrosis usually suffer the respiratory tract infection with S. maltophilia, there were not any patients with cystic fibrosis in this study because of the extremely low prevalence of cystic fibrosis in South Korea.33
The limitations of our study include the retrospective design with data from a single center only and the non-standardization of protocols for antimicrobial sensitivity testing in S. maltophilia. The results of an AST performed by disc diffusion, an E-test, and agar dilution may vary and may not correlate with in vivo effectiveness.34 The British Society for Antimicrobial Chemotherapy (BSAC) recommends only disk diffusion and agar dilution testing for TMP-SMX, while the CLSI generally recommends only disk diffusion testing and agar dilution testing for TMP-SMX, levofloxacin, and minocycline.18 We performed AST using the most recent revision of CLSI recommendations, the guideline that is most widely available. We further assumed that transitions between CLSI and BSAC recommendations for the AST would not affect the results of our regression analysis.
In conclusion, appropriate restrictions on the use of levofloxacin, currently among the commonly used antibiotics for S. maltophilia infection, may be necessary to prevent the emergence of levofloxacin resistance in S. maltophilia. Further study of this resistance through molecular epidemiologic analysis may clarify these clinical findings.
Figures and Tables
 | Fig. 1Selection of S. maltophilia isolates and patients. SMP, Stenotrophomonas maltophilia; AST, antimicrobial susceptibility test; S, susceptible; R, resistant. |
Table 1
Clinical Characteristics of the Study Population and Subgroups SS, Defined as the Maintenance of Levofloxacin Susceptibility, and SR, Defined as a Change from Levofloxacin Susceptibility to Resistance
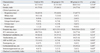
Table 2
Total Duration of Systemic Antibiotic Exposure within 3 Months from Identification of the Index Isolate in Patient Groups SS Was Defined as the Maintenance of Levofloxacin Susceptibility and SR Was Defined as a Change from Levofloxacin Susceptibility to Resistance
References
1. Garazi M, Singer C, Tai J, Ginocchio CC. Bloodstream infections caused by Stenotrophomonas maltophilia: a seven-year review. J Hosp Infect. 2012; 81:114–118.

2. Brooke JS. Stenotrophomonas maltophilia: an emerging global opportunistic pathogen. Clin Microbiol Rev. 2012; 25:2–41.

3. Fihman V, Le Monnier A, Corvec S, Jaureguy F, Tankovic J, Jacquier H, et al. Stenotrophomonas maltophilia--the most worrisome threat among unusual non-fermentative gram-negative bacilli from hospitalized patients: a prospective multicenter study. J Infect. 2012; 64:391–398.

4. Araoka H, Fujii T, Izutsu K, Kimura M, Nishida A, Ishiwata K, et al. Rapidly progressive fatal hemorrhagic pneumonia caused by Stenotrophomonas maltophilia in hematologic malignancy. Transpl Infect Dis. 2012; 14:355–363.

5. Falagas ME, Kastoris AC, Vouloumanou EK, Rafailidis PI, Kapaskelis AM, Dimopoulos G. Attributable mortality of Stenotrophomonas maltophilia infections: a systematic review of the literature. Future Microbiol. 2009; 4:1103–1109.

6. Sanchez MB, Hernandez A, Martinez JL. Stenotrophomonas maltophilia drug resistance. Future Microbiol. 2009; 4:655–660.
7. Abbott IJ, Slavin MA, Turnidge JD, Thursky KA, Worth LJ. Stenotrophomonas maltophilia: emerging disease patterns and challenges for treatment. Expert Rev Anti Infect Ther. 2011; 9:471–488.

8. Toleman MA, Bennett PM, Bennett DM, Jones RN, Walsh TR. Global emergence of trimethoprim/sulfamethoxazole resistance in Stenotrophomonas maltophilia mediated by acquisition of sul genes. Emerg Infect Dis. 2007; 13:559–565.

9. Chang LL, Lin HH, Chang CY, Lu PL. Increased incidence of class 1 integrons in trimethoprim/sulfamethoxazole-resistant clinical isolates of Stenotrophomonas maltophilia. J Antimicrob Chemother. 2007; 59:1038–1039.

10. Gales AC, Jones RN, Forward KR, Liñares J, Sader HS, Verhoef J. Emerging importance of multidrug-resistant Acinetobacter species and Stenotrophomonas maltophilia as pathogens in seriously ill patients: geographic patterns, epidemiological features, and trends in the SENTRY Antimicrobial Surveillance Program (1997-1999). Clin Infect Dis. 2001; 32:Suppl 2. S104–S113.
11. Sader HS, Jones RN. Antimicrobial susceptibility of uncommonly isolated non-enteric Gram-negative bacilli. Int J Antimicrob Agents. 2005; 25:95–109.

12. Farrell DJ, Sader HS, Jones RN. Antimicrobial susceptibilities of a worldwide collection of Stenotrophomonas maltophilia isolates tested against tigecycline and agents commonly used for S. maltophilia infections. Antimicrob Agents Chemother. 2010; 54:2735–2737.

13. Nicodemo AC, Paez JI. Antimicrobial therapy for Stenotrophomonas maltophilia infections. Eur J Clin Microbiol Infect Dis. 2007; 26:229–237.

14. Korakianitis I, Mirtsou V, Gougoudi E, Raftogiannis M, Giamarellos-Bourboulis EJ. Post-antibiotic effect (PAE) of moxifloxacin in multidrug-resistant Stenotrophomonas maltophilia. Int J Antimicrob Agents. 2010; 36:387–389.

15. Weiss K, Restieri C, De Carolis E, Laverdière M, Guay H. Comparative activity of new quinolones against 326 clinical isolates of Stenotrophomonas maltophilia. J Antimicrob Chemother. 2000; 45:363–365.

16. Valdezate S, Vindel A, Loza E, Baquero F, Cantón R. Antimicrobial susceptibilities of unique Stenotrophomonas maltophilia clinical strains. Antimicrob Agents Chemother. 2001; 45:1581–1584.

17. Garrison MW, Anderson DE, Campbell DM, Carroll KC, Malone CL, Anderson JD, et al. Stenotrophomonas maltophilia: emergence of multidrug-resistant strains during therapy and in an in vitro pharmacodynamic chamber model. Antimicrob Agents Chemother. 1996; 40:2859–2864.

18. CLSI. Performance standards for antimicrobial susceptibility testing; twenty-first informational supplement. M100-S21. Wayne, PA: Clinical and Laboratory Standard Institute;2011.
19. Hernández A, Maté MJ, Sánchez-Díaz PC, Romero A, Rojo F, Martínez JL. Structural and functional analysis of SmeT, the repressor of the Stenotrophomonas maltophilia multidrug efflux pump SmeDEF. J Biol Chem. 2009; 284:14428–14438.

20. Zhang L, Li XZ, Poole K. Multiple antibiotic resistance in Stenotrophomonas maltophilia: involvement of a multidrug efflux system. Antimicrob Agents Chemother. 2000; 44:287–293.

21. Gordon NC, Wareham DW. Novel variants of the Smqnr family of quinolone resistance genes in clinical isolates of Stenotrophomonas maltophilia. J Antimicrob Chemother. 2010; 65:483–489.

22. Cheng AF, Li MK, Ling TK, French GL. Emergence of ofloxacin-resistant Citrobacter freundii and Pseudomonas maltophilia after ofloxacin therapy. J Antimicrob Chemother. 1987; 20:283–285.

23. Ansari SR, Hanna H, Hachem R, Jiang Y, Rolston K, Raad I. Risk factors for infections with multidrug-resistant Stenotrophomonas maltophilia in patients with cancer. Cancer. 2007; 109:2615–2622.

24. Spanik S, Krupova I, Trupl J, Kunová A, Novotny J, Mateicka F, et al. Bacteremia due to multiresistant gram-negative bacilli in neutropenic cancer patients: a case-controlled study. J Infect Chemother. 1999; 5:180–184.

25. Rodríguez-Martínez JM, Cano ME, Velasco C, Martínez-Martínez L, Pascual A. Plasmid-mediated quinolone resistance: an update. J Infect Chemother. 2011; 17:149–182.

26. Araoka H, Baba M, Yoneyama A. Risk factors for mortality among patients with Stenotrophomonas maltophilia bacteremia in Tokyo, Japan, 1996-2009. Eur J Clin Microbiol Infect Dis. 2010; 29:605–608.

27. Gülmez D, Hasçelik G. Stenotrophomonas maltophilia: antimicrobial resistance and molecular typing of an emerging pathogen in a Turkish university hospital. Clin Microbiol Infect. 2005; 11:880–886.

28. Lai CH, Chi CY, Chen HP, Chen TL, Lai CJ, Fung CP, et al. Clinical characteristics and prognostic factors of patients with Stenotrophomonas maltophilia bacteremia. J Microbiol Immunol Infect. 2004; 37:350–358.
29. Tseng CC, Fang WF, Huang KT, Chang PW, Tu ML, Shiang YP, et al. Risk factors for mortality in patients with nosocomial Stenotrophomonas maltophilia pneumonia. Infect Control Hosp Epidemiol. 2009; 30:1193–1202.

30. Alonso A, Sanchez P, Martínez JL. Stenotrophomonas maltophilia D457R contains a cluster of genes from gram-positive bacteria involved in antibiotic and heavy metal resistance. Antimicrob Agents Chemother. 2000; 44:1778–1782.

31. Sánchez MB, Martínez JL. SmQnr contributes to intrinsic resistance to quinolones in Stenotrophomonas maltophilia. Antimicrob Agents Chemother. 2010; 54:580–581.

32. Sánchez MB, Martínez JL. Differential epigenetic compatibility of qnr antibiotic resistance determinants with the chromosome of Escherichia coli. PLoS One. 2012; 7:e35149.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download