Abstract
Purpose
The serum levels of soluble triggering receptor expressed on myeloid cells-1 (sTREM-1) have recently been shown to be correlated highly with disease activity in patients with intestinal Behçet's disease (BD). However, it remains unclear whether sTREM-1 levels reflect endoscopic activity in intestinal BD. This study aimed to evaluate the correlation of sTREM-1 levels with endoscopic activity in intestinal BD.
Materials and Methods
A total of 84 patients with intestinal BD were enrolled. Endoscopic activity was compared with sTREM-1 levels as well as other laboratory findings, including erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP).
Results
sTREM-1 levels were significantly increased in intestinal BD patients compared with controls (37.98±27.09 pg/mL vs. 16.65±7.76 pg/mL, p=0.002), however, there was no difference between endoscopically quiescent and active diseases (43.53±24.95 pg/mL vs. 42.22±32.68 pg/mL, p=0.819). Moreover, serum sTREM-1 levels did not differ in terms of number, shape, depth, size, margin, or type of ulcer in patients with intestinal BD. However, mean ESR and CRP levels in patients with active disease were significantly higher than those in patients with quiescent disease (p=0.001, p<0.001, respectively). In addition, endoscopic activity scores for intestinal BD were correlated significantly with both CRP levels (γ=0.329) and ESR (γ=0.298), but not with sTREM-1 levels (γ=0.166).
Intestinal Behçet's disease (BD), generally considered as a type of inflammatory bowel disease (IBD), has an unpredictable disease course with exacerbations and remission.1 Therefore, the timely objective assessment of disease activity is indispensable in patients with intestinal BD to administer appropriate therapies and monitor the effect of treatment. In IBD, along with the clinical parameters, endoscopy is traditionally used to measure disease activity by directly evaluating the severity and extent of mucosal lesions.2,3 As mucosal healing has emerged as a key treatment goal in IBD,4,5 the role of endoscopy in monitoring disease activity has been highlighted. However, because endoscopy is an invasive and expensive procedure, non-invasive, surrogate biomarkers are also actively being explored to predict the degree of inflammation and monitor disease activity.6,7 Among such biomarkers, erythrocyte sedimentation rate (ESR) and serum concentrations of C-reactive protein (CRP) have been the most studied, but these biomarkers have shown only a modest performance yet.
Although intestinal BD shares many clinical features with IBD and treatment is largely based on the strategies of IBD, recurrence and surgical intervention rates are reported to be even higher in patients with intestinal BD than with Crohn's disease (CD).8 In this regard, therefore, there is a critical need to develop accurate tools to measure disease activity for intestinal BD. However, data regarding the objective means of determining disease activity of intestinal BD are extremely limited. We have recently reported the disease activity index for intestinal BD that assesses clinical activity9 and an endoscopic severity model, respectively,10 but no biomarkers were identified to be associated with intestinal BD.
Triggering receptor expressed on myeloid cells-1 (TREM-1) is a member of the immunoglobulin superfamily and is constitutively expressed on the surfaces of neutrophils and monocyte/macrophages; it triggers the synthesis and secretion of proinflammatory cytokines.11 Levels of soluble TREM-1 (sTREM-1) were found to be elevated, not only in acute inflammatory conditions such as pneumonia and sepsis,12,13 but also in chronic inflammatory disorders such as rheumatoid arthritis.14 Moreover, recent studies found that TREM-1 was upregulated in the inflamed mucosa of patients with IBD15 and sTREM-1 correlated with both clinical and endoscopic disease activities of ulcerative colitis (UC),16,17,18 implicating the potential role of sTREM-1 to assess disease activity in IBD patients. Because we earlier showed that sTREM-1 correlated better with clinical disease activity than either ESR or CRP,19 in the present study, we aimed to evaluate whether sTREM-1 also could predict endoscopic activity in intestinal BD compared to ESR or CRP.
Between July 2006 and December 2009, 84 patients with intestinal BD seen at Severance Hospital, Yonsei University, Seoul, Korea were enrolled in our study. Intestinal BD was diagnosed according to the previously established criteria, based on colonoscopic features and clinical manifestations, using a modified Delphi process.20 Patients classified as definite, probable, and suspected were included in this study. The exclusion criteria were as follows: 1) coexisting infectious or ischemic colitis, 2) other coexisting localized or systemic infections, 3) a diagnosis of liver cirrhosis, 4) a history of administration of anti-tumor necrosis factor-α (TNF-α) antibodies, and 5) a history of bowel resection. The control group included six healthy individuals who had no gastrointestinal symptoms, took no regular medications, and had a normal complete blood count and a normal biochemical profile, including ESR and CRP levels. All control subjects underwent colonoscopy for routine check-up, which revealed grossly normal findings.
At the time of colonoscopy, sTREM-1 levels as well as other laboratory findings including ESR and CRP were measured and compared with endoscopic activity. Serum sTREM-1 levels were measured using a commercialized, specific, enzyme-linked immunosorbent assay kit (R&D systems, Minneapolis, MN, USA) according to the manufacturer's protocol. Briefly, samples were incubated in wells coated with a TREM-1-specific monoclonal antibody for 2 h at room temperature (RT). After washing the wells four times, a peroxidase-conjugated anti-TREM-1 polyclonal antibody was added to the wells, and the samples were incubated for 2 h at RT. After washing an additional four times, the substrate solution was added, and after a 30-min incubation at RT, the stop solution was added to terminate the enzyme reaction. Absorbance was measured at 450 nm using a microplate reader, with the wavelength corrected by measuring and comparing the absorbance to 540 nm. Measurements were performed in triplicate. ESR was determined from whole blood according to the Westergren method (Alifax, Padova, Italy). Serum CRP concentrations were assessed using a nephelometric method (Beckman Coulter, Fullerton, CA, USA). This study was approved by the Institutional Review Board of Severance Hospital and all subjects provided written informed consent.
Endoscopic disease activity was divided into two categories as quiescent and active disease according to the ulcer status to investigate the relationship between endoscopic activity and biomarkers. In patients with active ulcers, variable endoscopic factors, including location, number, depth, shape, and size of ulcer, were measured to assess their correlations with sTREM-1. In addition, endoscopic activity was adjunctively determined in patients with intestinal BD using a recently developed, novel endoscopic severity model.10
Variables are expressed as means±standard deviation (SD). Continuous variables were compared using an independent t test or a Mann-Whitney U test, and categorical variables were compared with a chi-square test or Fisher's exact test. Associations between endoscopic disease activity and sTREM-1, CRP, or ESR were assessed using Spearman's rank correlation coefficients (γ) for nonparametric correlations. The multivariate logistic analysis was performed to determine biomarkers associated with endoscopically active disease. Serial receiver operating characteristic (ROC) curves [±95% confidence interval (CI)] were used to define the diagnostic accuracy of sTREM-1, ESR, and CRP to predict endoscopically active disease. p<0.05 was considered statistically significant. All statistical analyses were performed using SPSS software (ver. 18.0; SPSS Inc., Chicago, IL, USA).
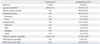
Table 1 shows the clinical characteristics and laboratory findings of the 84 intestinal BD patients and 6 control subjects enrolled in the study. There were no statistically significant differences between the two groups with respect to demographic properties such as gender or age. Of the 84 patients with intestinal BD, 17 (20.2%) showed quiescent disease activity, 27 (32.1%) had mild activity, 26 (31.0%) had moderate activity, and 14 (16.7%) showed severe disease activity
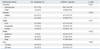
The number of patients with endoscopically active disease was 61 (72.6%) and that with quiescent disease was 23 (27.4%). The mean CRP and ESR levels in patients with active disease were significantly higher than those in patients with quiescent disease (1.60±2.28 mg/dL vs. 0.31±0.23 mg/dL, p<0.001 and 36.18±24.62 mm/h vs. 20.70±15.54 mm/h, p=0.001, respectively), whereas the mean sTREM-1 levels in patients with active disease did not differ from those in patients with quiescent disease (43.53±24.95 pg/mL vs. 42.22±32.68 pg/mL, p=0.819) (Table 2). Endoscopic activity score for intestinal BD correlated significantly with both CRP levels (γ=0.329, p=0.002) and ESR (γ=0.298, p=0.006), but not with sTREM-1 levels (γ=0.166, p=0.132). Among these, CRP levels showed the highest correlation (Supplementary Fig. 1, only online). The results of multiple linear regression analyses for the three biomarkers associated with endoscopically active disease are shown in Table 3. Only CRP level was independently correlated with endoscopic activity after adjusting for other biomarkers (OR 7.08, CI 1.24-40.33, p=0.027).
Using ROC curves, CRP had the best discriminatory power [area under receiver operating characteristic (AUROC) 0.737, p=0.001] in detecting endoscopically active disease, followed by ESR (AUROC 0.695, p=0.006) and sTREM-1 (AUROC 0.553, p=0.455) (Supplementary Table 1, only online).
To determine whether serum sTREM-1 levels differ according to endoscopic factors, we analyzed 61 patients with endoscopically active disease. Serum sTREM-1 levels did not differ in terms of number, shape, depth, size, margin, or type of ulcer. However, serum sTREM-1 levels in patients with ileocecal area ulcer were significantly lower than those in patients with colonic ulcers (40.13±23.95 vs. 55.90±25.26, p=0.021) (Table 4).
Biomarkers have been investigated in IBD for assessment of disease activity, prediction of disease course and outcome, and monitoring effects of therapy. In addition to ESR and CRP, fecal markers have also been studied recently in IBD because they would be more specific in the absence of enteric infection.6,7 However, no single marker has so far been proven to be ideal, since ESR takes several days to decline due to long half lives even when rapid clinical improvement occurs,7 and CRP correlates less well with disease activity in UC compared to CD.21 Considering unpredictable disease flares and poor overall prognosis in intestinal BD,8 assessment of disease activity is inevitable to decide an appropriate treatment plan and evaluate response. Although there are few studies to show high CRP level as a poor prognostic factor22 and association of anti-Saccharomyces cerevisiae antibody positivity with increased surgical rate,23 data on which biomarkers could predict disease activity of intestinal BD are extremely limited.
Recently, as increased levels of sTREM-1 were observed in patients with moderate or severe IBD,15,16 sTREM-1 was evaluated as a promising biomarker to monitor disease activity in IBD. sTREM-1 levels were correlated most accurately with clinical and endoscopic disease activities in UC regardless of disease extent.17,18 Jung, et al.19 also showed the most close correlation between sTREM-1 and clinical activity among serum biomarkers, including CRP and ESR in intestinal BD. Because there are no decisive conclusions about the role of sTREM-1 in IBD, we aimed to identify whether sTREM-1 was elevated in intestinal BD with endoscopically active disease and whether sTREM-1 could be a reliable marker to assess disease activity in patients with intestinal BD.
In the present study, we demonstrated that sTREM-1 levels were significantly upregulated in intestinal BD patients compared to normal controls, the finding similar to data previously reported in IBD.15,17,24 Our findings thus suggest that sTREM-1 might be involved in the pathogenesis of intestinal BD, leading to increased proinflammatory mediators and contributing to sustained inflammation and disease exacerbation. However, among patients with intestinal BD, sTREM-1 was also elevated in endoscopically quiescent disease to similar levels as in active disease. Similarly, two interesting articles reveal the disparities in the relationship of sTREM-1 level with disease activity in IBD.24,25 One of the possible explanations is that, even in patients with quiescent disease, subclinical inflammation may be enough to cause bacterial translocation that may initiate the release of sTREM-1. It is also possible that since up to 50% of patients with intestinal BD have frequent intestinal complications, such as bowel perforation, fistula, and abscess formation,26,27 extraintestinal manifestations can affect sTREM-1 levels irrespective of endoscopic activity. Because there were no statistical differences in the intestinal complications and extraintestinal manifestations between the two groups in our study (data not shown), it is possible that sTREM-1 might be more specific and sensitive for intestinal mucosa than either CRP or ESR in reflecting subclinical inflammation of intestinal BD. Such findings imply that sTREM-1 could play a role in discriminating intestinal BD from other functional disorders like irritable bowel syndrome (IBS), which often share clinical similarities and cause difficulties in diagnosis.28,29 Further studies are needed to identify the potential value of sTREM-1 as a differential diagnostic marker.
In patients with intestinal BD, we did not find any correlation between sTREM-1 levels and endoscopic activity, which was contradictory to our previous study that showed the highest correlation between sTREM-1 and clinical activity.19 The reasons for this discrepancy are not clear, nevertheless, it might be attributable to the different release pattern of TREM-1. Previously, S100A9 protein, which expression levels were significantly correlated with TREM-1 levels,24 was found to be lower in ileal CD than colonic inflammation because of degradation during the passage from the ileum, which should be further tested.30 Since most patients with intestinal BD have lesions in the ileocecal region,8 sTREM-1 levels might not accurately reflect disease activity in intestinal BD. These hypotheses were verified in this study in that higher sTREM-1 levels were observed in other colonic areas than ileocecal areas in patients with endoscopically active disease. Furthermore, we previously reported that the correlation between endoscopic and clinical activity was unexpectedly weak,10 possibly explaining these contradictory results. Further research is required to evaluate the relationship between sTREM-1 and disease activity in intestinal BD.
In contrast to the results regarding sTREM-1, both CRP and ESR levels were found to be correlated highly with endoscopic activity in intestinal BD patients. Among them, CRP showed the best performance in assessing endoscopic activity in intestinal BD. There are limited number of studies demonstrating that patients with elevated CRP levels have an increased risk of recurrence and repeat surgery,22,31 and that CRP and ESR levels are correlated with clinical activity in intestinal BD.19 More studies are necessary to validate the role of CRP.
Our study has several limitations. First, we could not investigate whether sTREM-1 could predict disease relapse, because long-term follow-up data were not measured. Second, we could not identify the association between sTREM-1 and changes in endoscopic activity, because serial sTREM-1 levels according to various clinical courses were not determined in this study. Finally, standardized methods to assess disease activity for the use in predicting disease course and monitoring treatment responses in intestinal BD are yet to be established. Although we recently reported an endoscopic severity model,10 it has yet to be validated and be reproduced in the prospective study. In addition, further studies are warranted to develop a reliable tool to evaluate disease activity that can precisely predict the prognosis of intestinal BD.
In conclusion, the present study demonstrated that sTREM-1 levels were significantly increased in intestinal BD patients compared to normal controls, but did not differ between endoscopically quiescent and active disease. In addition, serum sTREM-1 levels did not show any significant correlation with endoscopic activity, unlike CRP levels or ESR, in patients with intestinal BD. Taken together, we suggest that serum sTREM-1 level might not be an appropriate biomarker for the assessment of endoscopic activity in intestinal BD and further investigations are mandatory to identify a reliable marker to predict both clinical and endoscopic disease activities in intestinal BD.
Figures and Tables
 | Fig. 1Serum sTREM-1 levels were significantly higher in patients with intestinal BD than in controls. BD, Behçet's disease; sTREM-1, soluble triggering receptor expressed on myeloid cells-1. |
Table 2
Comparison of Serum sTREM-1, CRP, and ESR Levels among the Patients with Endoscopically Inactive or Active Disease

ACKNOWLEDGEMENTS
This work was supported by a grant of the Korean Health Technology R&D Project, Ministry of Health & Welfare, Republic of Korea (grant number A120176).
This study was also supported by a faculty research grant of Yonsei University College of Medicine for 2012 (4-2012-0680).
Notes
References
2. Allez M, Lémann M. Role of endoscopy in predicting the disease course in inflammatory bowel disease. World J Gastroenterol. 2010; 16:2626–2632.

3. Cheon JH, Kim WH. Recent advances of endoscopy in inflammatory bowel diseases. Gut Liver. 2007; 1:118–125.

4. Frøslie KF, Jahnsen J, Moum BA, Vatn MH. IBSEN Group. Mucosal healing in inflammatory bowel disease: results from a Norwegian population-based cohort. Gastroenterology. 2007; 133:412–422.

5. Neurath MF, Travis SP. Mucosal healing in inflammatory bowel diseases: a systematic review. Gut. 2012; 61:1619–1635.

6. Vermeire S, Van Assche G, Rutgeerts P. Laboratory markers in IBD: useful, magic, or unnecessary toys? Gut. 2006; 55:426–431.

7. Desai D, Faubion WA, Sandborn WJ. Review article: biological activity markers in inflammatory bowel disease. Aliment Pharmacol Ther. 2007; 25:247–255.

8. Grigg EL, Kane S, Katz S. Mimicry and deception in inflammatory bowel disease and intestinal behçet disease. Gastroenterol Hepatol (N Y). 2012; 8:103–112.
9. Cheon JH, Han DS, Park JY, Ye BD, Jung SA, Park YS, et al. Development, validation, and responsiveness of a novel disease activity index for intestinal Behçet's disease. Inflamm Bowel Dis. 2011; 17:605–613.

10. Lee HJ, Kim YN, Jang HW, Jeon HH, Jung ES, Park SJ, et al. Correlations between endoscopic and clinical disease activity indices in intestinal Behcet's disease. World J Gastroenterol. 2012; 18:5771–5778.

11. Bouchon A, Dietrich J, Colonna M. Cutting edge: inflammatory responses can be triggered by TREM-1, a novel receptor expressed on neutrophils and monocytes. J Immunol. 2000; 164:4991–4995.

12. Gibot S, Cravoisy A, Levy B, Bene MC, Faure G, Bollaert PE. Soluble triggering receptor expressed on myeloid cells and the diagnosis of pneumonia. N Engl J Med. 2004; 350:451–458.

13. Gibot S, Le Renard PE, Bollaert PE, Kolopp-Sarda MN, Béné MC, Faure GC, et al. Surface triggering receptor expressed on myeloid cells 1 expression patterns in septic shock. Intensive Care Med. 2005; 31:594–597.

14. Collins CE, La DT, Yang HT, Massin F, Gibot S, Faure G, et al. Elevated synovial expression of triggering receptor expressed on myeloid cells 1 in patients with septic arthritis or rheumatoid arthritis. Ann Rheum Dis. 2009; 68:1768–1774.

15. Schenk M, Bouchon A, Seibold F, Mueller C. TREM-1--expressing intestinal macrophages crucially amplify chronic inflammation in experimental colitis and inflammatory bowel diseases. J Clin Invest. 2007; 117:3097–3106.

16. Tzivras M, Koussoulas V, Giamarellos-Bourboulis EJ, Tzivras D, Tsaganos T, Koutoukas P, et al. Role of soluble triggering receptor expressed on myeloid cells in inflammatory bowel disease. World J Gastroenterol. 2006; 12:3416–3419.

17. Park JJ, Cheon JH, Kim BY, Kim DH, Kim ES, Kim TI, et al. Correlation of serum-soluble triggering receptor expressed on myeloid cells-1 with clinical disease activity in inflammatory bowel disease. Dig Dis Sci. 2009; 54:1525–1531.

18. Jung YS, Park JJ, Kim SW, Hong SP, Kim TI, Kim WH, et al. Correlation between soluble triggering receptor expressed on myeloid cells-1 (sTREM-1) expression and endoscopic activity in inflammatory bowel diseases. Dig Liver Dis. 2012; 44:897–903.

19. Jung YS, Kim SW, Yoon JY, Lee JH, Jeon SM, Hong SP, et al. Expression of a soluble triggering receptor expressed on myeloid cells-1 (sTREM-1) correlates with clinical disease activity in intestinal Behcet's disease. Inflamm Bowel Dis. 2011; 17:2130–2137.

20. Cheon JH, Kim ES, Shin SJ, Kim TI, Lee KM, Kim SW, et al. Development and validation of novel diagnostic criteria for intestinal Behçet's disease in Korean patients with ileocolonic ulcers. Am J Gastroenterol. 2009; 104:2492–2499.

21. Solem CA, Loftus EV Jr, Tremaine WJ, Harmsen WS, Zinsmeister AR, Sandborn WJ. Correlation of C-reactive protein with clinical, endoscopic, histologic, and radiographic activity in inflammatory bowel disease. Inflamm Bowel Dis. 2005; 11:707–712.

22. Jung YS, Yoon JY, Lee JH, Jeon SM, Hong SP, Kim TI, et al. Prognostic factors and long-term clinical outcomes for surgical patients with intestinal Behcet's disease. Inflamm Bowel Dis. 2011; 17:1594–1602.

23. Choi CH, Kim TI, Kim BC, Shin SJ, Lee SK, Kim WH, et al. Anti-Saccharomyces cerevisiae antibody in intestinal Behçet's disease patients: relation to clinical course. Dis Colon Rectum. 2006; 49:1849–1859.

24. Saurer L, Rihs S, Birrer M, Saxer-Seculic N, Radsak M, Mueller C, et al. Elevated levels of serum-soluble triggering receptor expressed on myeloid cells-1 in patients with IBD do not correlate with intestinal TREM-1 mRNA expression and endoscopic disease activity. J Crohns Colitis. 2012; 6:913–923.

25. Billioud V, Gibot S, Massin F, Oussalah A, Chevaux JB, Williet N, et al. Plasma soluble triggering receptor expressed on myeloid cells-1 in Crohn's disease. Dig Liver Dis. 2012; 44:466–470.

26. Kasahara Y, Tanaka S, Nishino M, Umemura H, Shiraha S, Kuyama T. Intestinal involvement in Behçet's disease: review of 136 surgical cases in the Japanese literature. Dis Colon Rectum. 1981; 24:103–106.
27. Moon CM, Cheon JH, Shin JK, Jeon SM, Bok HJ, Lee JH, et al. Prediction of free bowel perforation in patients with intestinal Behçet's disease using clinical and colonoscopic findings. Dig Dis Sci. 2010; 55:2904–2911.

28. Ginsburg PM, Bayless TM. How can IBD be distinguished from IBS? Inflamm Bowel Dis. 2008; 14:Suppl 2. S152–S154.

29. Grundmann O, Yoon SL. Irritable bowel syndrome: epidemiology, diagnosis and treatment: an update for health-care practitioners. J Gastroenterol Hepatol. 2010; 25:691–699.

Supplementary Material
Supplementary Fig. 1
Endoscopic activity score for intestinal BD was correlated significantly with both CRP level (γ=0.329, p=0.002) and ESR (γ=0.298, p=0.006), but not with sTREM-1 level (γ=0.166, p=0.132). Correlations between variables were analyzed using Spearman's rank correlation. sTREM-1, soluble triggering receptor expressed on myeloid cells-1; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download