Abstract
Purpose
Due to the seroepidemiological shift in hepatitis A (HA), its severity, mortality, and complications have increased in recent years. Thus, the aim of this study was to identify predictive factors associated with poor prognosis among patients with HA.
Materials and Methods
A total of 304 patients with HA admitted to our institution between July 2009 and June 2011 were enrolled consecutively. Patients with complications defined as acute liver failure (ALF) were evaluated, and mortality was defined as death or liver transplantation.
Results
The mean age of patients (204 males, 100 females) was 32 years. Eighteen (5.9%) patients had progressed to ALF. Of the patients with ALF, 10 patients (3.3%) showed spontaneous survival while 8 (2.6%) died or underwent liver transplantation. Multivariate regression analysis showed that Model for End-Stage Liver Disease (MELD) and systemic inflammatory response syndrome (SIRS) scores were significant predictive factors of ALF. Based on receiver operating characteristics (ROC) analysis, a MELD ≥23.5 was significantly more predictive than a SIRS score ≥3 (area under the ROC: 0.940 vs. 0.742, respectively). In addition, of patients with a MELD score ≥23.5, King's College Hospital criteria (KCC) and SIRS scores were predictive factors associated with death/transplantation in multivariate analysis.
The hepatitis A virus (HAV) is the most common cause of acute hepatitis worldwide.1 Less than 30% of infected young children show symptomatic hepatitis, while -80% of infected adults manifest severe acute hepatitis.2,3 Recently, we observed an increased incidence of hepatitis A (HA) in adults. This is mostly due to the seroepidemiological shifts associated with rapid economic development. With the increased incidence of HA in adults, the severity, mortality and complications are increasing compared with previous periods. In fact, in Korea, there was an outbreak of HA in 2008; it has become a very important public health problem.4
The main complication of HAV infection is acute liver failure (ALF). Although HAV-related ALF spontaneously resolves more frequently than ALF of another origin, as many as 50% of HAV patients with ALF may die or require emergency liver transplantation.5,6 In addition, HAV-related ALF progresses faster than ALF caused by other etiologies.7 Thus, it is both difficult and important to identify patients in need of urgent liver transplantation. Traditionally, the Model for End-Stage Liver Disease (MELD) and King's College Hospital criteria (KCC) scores have been used for predicting ALF prognosis.8 In recent years, however, various prognostic indicators, including systemic inflammatory response syndrome (SIRS), have been proposed for the evaluation of ALF.9 However, as mentioned earlier, since rapid disease progression and prognosis of HAV-related ALF is different than other origin-related ALF, alternative or additional prognostic markers are required.
Red blood cell distribution width (RDW) originally represented the size variation of all red blood cells and was routinely performed as part of a complete blood cell count.10 Recently, several studies have demonstrated that RDW can serve as a novel, independent predictor of prognosis in patients with cardiovascular diseases, acute kidney injury and sepsis.11,12,13 These conditions are often present in patients with liver disease, correlate with the severity of the disease, and are associated with a worse prognosis.14 However, to our knowledge, the relevance of RDW to HAV infection prognosis and ALF has rarely been addressed.
The aim of this investigation was to reassess factors predicting ALF development and mortality in patients with HA by assessing several clinical factors, including prognostic models.
Between July 2009 and June 2011, a total of 319 patients with HA diagnosed at Severance Hospital, Yonsei University College of Medicine, Seoul, Korea, were consecutively enrolled in this study. Of these, patients with the following conditions were excluded: 1) under 18 years old, 2) history of excessive alcohol intake (more than 50 g of alcohol per day over 10 years),15 3) referred patients with previously diagnosed ALF, and 4) an incomplete medical record. After exclusions, a total of 304 patients were included in the analyses. Data were collected prospectively and entered into a dedicated database. Demographic data, baseline laboratory data, and underlying medical problems, such as diabetes mellitus (DM) and chronic viral hepatitis, at diagnosis were recorded. The following variables were recorded at the time of hospital admission: body temperature, pulse, hemoglobin, RDW, white blood cell (WBC) counts, platelet counts, prothrombin time (international normalized ratio, INR), total bilirubin, alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase, albumin, and creatinine.
All HA patients received standard symptomatic treatment. Nevertheless, when ALF developed, we performed an artificial supportive therapy such as molecular adsorbent recirculating system (MARS®; Gambro, Lakewood, CO, USA) for hepatic function recovery or as a bridge to liver transplantation.
Our study protocol was consistent with the ethical guidelines of the 1975 Declaration of Helsinki and was approved by the independent Institutional Review Board of our institute.
The diagnosis of HA was based on the detection of serum IgM antibodies against HAV measured by an enzyme immunoassay (Abbott Laboratories, North Chicago, IL, USA). The clinical diagnosis of ascites was made by the presence of shifting dullness of fluid in the abdomen or the presence of fluid thrill. Ultrasound examination of the abdomen was performed when clinical signs for ascites were ambiguous. Hepatic encephalopathy (HEP) was graded from 1 to 4 according to the West-Hevan criteria.16 Patients with complications defined as ALF were evaluated, and mortality was defined as death or liver transplantation. ALF was defined as coagulation abnormality (INR ≥1.5) and any degree of HEP.17 The definition of acute renal failure was based on an increase in serum creatinine >0.5 mg/dL over the baseline value, or an increase of more than 50% over the baseline value.18
The clinical parameters and first available laboratory tests at diagnosis were used to calculate the MELD score, KCC and SIRS score. The MELD score was calculated using the website calculator {(http://www.unos.org/resources/meld-PeldCalculator.asp); MELD score=3.8×log [total bilirubin (mg/dL)]+11.2×log (INR)+9.6×log [creatinine (mg/dL)]+6.43; any value less than one was given a value of 1}.19 The KCC for non-acetaminophen-related ALF was defined as follows: A) prothrombin time >100 seconds (INR >6.5) or B) if any three of the following were present: 1) age <10 or >40; 2) cause: non-A, non-B hepatitis/idiosyncratic drug reaction, 3) jaundice to encephalopathy >7 days, 4) prothrombin time >50 s (INR >3.5), or 5) serum bilirubin >17.5 mg/dL.20 The presence of SIRS was defined as two or more of the followings: temperature <36℃ or >38℃, heart rate >90 beats/min, leukocyte count <4×103/mm3 or >12×103/mm3, and tachypnea >20 breaths/min or PaCO2 <4.3 kPa.5 The number of SIRS components was fulfilled upon admission. Each patient was given a SIRS score depending on the number of SIRS components fulfilled at diagnosis.21
Data are expressed as means±standard deviation or n (%) unless otherwise stated. Student's t-test was used to compare continuous variables, and the chi-square test (or Fisher's exact test) was used for categorical variables. Univariate and subsequent multivariate logistic regression analyses were used to determine independent predictors of ALF development. Odds ratios (ORs) and corresponding 95% confidence intervals (CIs) are indicated. Receiver operating characteristics (ROC) curves and area under the ROC (AUROC) were used to identify which variables had the best discrimination capacity to predict ALF development. All two-sided p values were considered significant if <0.05. Statistical analyses were performed using SPSS software (ver. 18.0; SPSS Inc., Chicago, IL, USA).
Baseline characteristics of the 304 HA patients are summarized in Table 1. The mean ages of the non-ALF (n=286, 94.1%) and ALF groups (n=18, 5.9%) were 32±8 and 34±8 years, respectively, and did not show significant difference (p=0.340). The proportion of males and mean body mass index (BMI) were not significantly different between the groups. The number of patients with DM was higher in the ALF group compared with the non-ALF group (p=0.016). Hepatitis B virus surface antigen positivity was 17 (6.0%) and 3 (16.7%), respectively. Total bilirubin, albumin, AST, ALT, prothrombin time (INR), creatinine and WBC count, and RDW ratio >13.5 were significantly higher in the ALF group (all p-values <0.05). Patients with ALF had higher MELD scores (34.78±9.30 vs. 13.87±6.29; p=0.001), fulfilled KCC (0 vs. 5; p=0.001), and SIRS scores (0.5±0.9 vs. 1.7±1.2; p=0.002) than patients without ALF.
Clinical outcomes are presented in Fig. 1. Of the 304 patients enrolled, 286 (94.1%) showed spontaneous recovery without ALF, whereas ALF occurred in 18 (5.9%) patients. Mean duration from the first clinical manifestation to ALF onset was 4.5±1.5 days. Of the ALF patients, 10 (3.3%) showed spontaneous survival while 8 (2.6%) died during hospitalization or underwent liver transplantation.
Table 2 shows the predictive indicators significantly associated with ALF development. On univariate logistic regression analysis, DM (p=0.004), ALT (0.008), albumin (p=0.001), RDW >13.5 (p=0.001), MELD score (p=0.001), KCC (p=0.001), and SIRS score (p=0.001) were significantly associated with ALF development. In stepwise multivariate analysis, only the MELD (OR 1.149, CI 1.055-1.250, p=0.001) and SIRS scores (OR 2.160, CI 1.094-4.267, p=0.027) showed significant correlation. The sensitivities, specificities, positive predictive values (PPV), and negative predictive values (NPV) are shown in Table 3. A MELD score ≥23.5 was superior to a SIRS score ≥3 in terms of predicting the development of complications (AUROC 0.940 vs. 0.742).
Data of patients with a MELD score ≥23.5 were analyzed with regard to mortality. Based on a stepwise multivariate analysis, KCC (OR 53.353, CI 1.735-1640.878, p=0.023) and SIRS score (OR 3.564, CI 1.152-11.030, p=0.027) showed a significant correlation (Table 4). In ROC analysis, a SIRS score ≥3 was superior to fulfilled KCC in predicting death/transplantation (AUROC 0.803 vs. 0.731). The sensitivity, specificity, PPV, and NPV of the SIRS score ≥3 were 87.5%, 73.1%, 50.0%, 95.0%, respectively.
ALF is a rare but potentially fatal complication of acute hepatitis for which liver transplantation is the only definitive therapy.22 In Asia, viral hepatitis has been a major cause of ALF, whereas acetaminophen has been the most common cause of ALF in western countries.23 ALF from HA occurs in -0.1-0.3% of all infections but increased significantly in 2008 in Korea due to increased HAV infection in adults along with decreased opportunity for HAV infection in younger individuals.24 Thus, HA is not just a benign disease that can be resolved by conservative treatment; it needs to be aware of the potential of morbidity and mortality.
Many clinical and investigational variables have been proposed worldwide to predict outcome of patients with HA, but their accuracy and feasibility are still under debate. Our study was carried out prospectively to identify factors influencing ALF development and mortality in HA patients by analyzing several clinical factors, including prognostic models. An interesting point of this study is that we investigated variable factors such as DM, BMI, RDW, and the SIRS score, which had not yet been applied to prediction of HA outcome. Recently, the SIRS score, the clinical manifestation of inflammation, was reported to be associated with the prognosis of ALF patients.25 Several studies have revealed that SIRS worsens the grade of HEP and increases the mortality rates as the number of SIRS fulfilled components increases. Rolando, et al.9 reported that mortality in ALF patients increases with a greater magnitude of SIRS in both acetaminophen and other etiologies. Moreover, Craig et al.26 showed that the SIRS score is useful for identifying patients with a poor prognosis in paracetamol-induced ALF and effective triage markers following a paracetamol overdose. Our present findings agree with those studies. The SIRS score was an effective predictor of ALF development in patients with HA, although the predictive power was lower (AUROC 0.742) than that of the MELD score (AUROC 0.940). In addition, the SIRS score is useful in predicting mortality in patients with high MELD scores (≥23.5), revealed by stepwise multivariate analysis. A SIRS score ≥3 is highly predictive of spontaneous survival; these patients also have a low risk of ALF development. As a result of its high negative predictive value (90.4%), the SIRS score may have a potential role as a 'gatekeeper' when considering patient transfer to a tertiary liver transplant center. However, its relatively lower sensitivity indicates that it cannot completely replace the MELD or KCC as definitive listing criteria.
The MELD score has been a validated predictive model of short-term mortality in patients with cirrhosis and has been used for ranking donor organ allocation in patients awaiting liver transplantation.27,28 Whether MELD is also useful in cases of ALF is controversial. Dhiman, et al.29 reported that the MELD score is less useful for accurately predicting ALF outcome due to acute viral hepatitis, whereas Katoonizadeh, et al.30 proposed that MELD has an excellent prognostic value in terms of predicting the outcome in adult patients with non-acetaminophen induced ALF. In the current study, the MELD score was a powerful tool for predicting ALF development in patients with HA compared to DM, ALT, albumin, RDW ≥13.5, and the SIRS score.
Recently, several studies showed that RDW can reflect the level of inflammation and predict mortality in critically ill patients.31 Although the mechanism of the association between RDW and mortality remains unclear, RDW is an attractive tool because it is inexpensive and easy to check. Thus, we attempted to evaluate the relevance of RDW to prognosis in HAV infections. In our study, a RDW ratio >13.5 was significantly more frequent in the ALF group (61% vs. 21.3%, p=0.001), although a RDW ≥13.5 lost its predictive power in stepwise multivariate analysis. MELD and SIRS scores are believed to have a too-strong effect on ALF development. However, this study was valuable because we evaluated RDW as a predictor of HA for the first time.
Previous studies have suggested that the presence of chronic hepatitis B or C and age are major risk factors for ALF development with acute HAV infection.32 In our study, however, in contrast to these reports, age and chronic hepatitis were not effective predictors of prognosis. Older age is classically related to greater morbidity and HA mortality; however, it is not clear as to whether this is directly due to liver damage or decomposition of underlying disease.33 Choi, et al.34 reported that patients >40 years of age did not show differences in clinical outcomes. Similar to these results, the population of this study ranged from 20-60 years of age, and ALF developed between 25-40 years of age. Although the incidence of HA-related ALF was higher in adults than in children, it did not increase with age in adults. Our results showed the highest incidence of HAV-related ALF in young adults of their thirties. Thus, age may not affect the prognosis of HA. To date, the impact of chronic hepatitis B on the clinical outcome of HA has remained controversial. These conflicting results may be due to missed severity of underlying hepatitis B virus (HBV) infection.35 In the current study, patients with neither cirrhosis nor high levels of HBV DNA (>2000 IU/mL) were identified. In this regard, our results are more reliable than those of other reports related to chronic hepatitis B and HA.
Three criticisms of this study can be made. First, the prevalence of ALF was higher due to the characteristics of this institute, a tertiary hospital with a liver transplant center, and some subjects were referred with no prior record of earlier conditions of the disease. Second, the number of subjects with ALF was small because of the nature of hepatitis A. Therefore, these issues limited our ability to unravel statistically significant differences. Third, many reports showed that HAV genotype and HAV load are associated with the severity of hepatitis A. However, analysis of HAV genotypes and viral quantification was not available in this study.
To cure more HA-related ALF patients, early liver transplantation is important, and prognostic factors for the prediction of ALF development are needed. In our study, higher MELD (≥23.5) and SIRS (≥3) scores appeared to be related to the development of ALF in patients with HA. Furthermore, in patients with a higher MELD score (≥23.5), those with a SIRS score ≥3 and who fulfilled KCC may need urgent liver transplantation. Thus, we believe that the SIRS score, in conjunction with MELD and KCC, may be important for determining the appropriate treatment strategy, including the timing of liver transplantation in HA-related ALF. Another important strategy against HAV infection is vaccination. Universal vaccination program would be advisable. A further prospective validation study is necessary.
Figures and Tables
Table 1
Baseline Characteristic of the Patients at Diagnosis According to Outcome
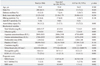
ALF, acute liver failure; HBsAg, hepatitis B virus surface antigen; anti HCV, anti hepatitis C virus; RDW, red blood cell distribution width; MELD, Model for End-Stage Liver Disease; KCC, King's College Hospital criteria; SIRS, systemic inflammatory response syndrome; INR, international normalized ratio.
Values are expressed as mean±SD or n (%).
ACKNOWLEDGEMENTS
This work was supported by The GlaxoSmithKline Research Fund of the Korean Association for the Study of the Liver (2014 GlaxoSmithKline Academic Scholarship of the Korean Association for the Study of the Liver). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
2. Kang CI, Choi CM, Park TS, Lee DJ, Oh MD, Choe KW. Incidence and seroprevalence of hepatitis A virus infections among young Korean soldiers. J Korean Med Sci. 2007; 22:546–548.

3. Jeong SH, Lee HS. Hepatitis A: clinical manifestations and management. Intervirology. 2010; 53:15–19.

4. Jung YK, Kim JH. [Epidemiology and clinical features of acute hepatitis A: from the domestic perspective]. Korean J Hepatol. 2009; 15:438–445.

5. Schiødt FV, Davern TJ, Shakil AO, McGuire B, Samuel G, Lee WM. Viral hepatitis-related acute liver failure. Am J Gastroenterol. 2003; 98:448–453.

6. Schiodt FV, Atillasoy E, Shakil AO, Schiff ER, Caldwell C, Kowdley KV, et al. Etiology and outcome for 295 patients with acute liver failure in the United States. Liver Transpl Surg. 1999; 5:29–34.

7. Kim JD, Choi JY, Park CH, Song MJ, Jang JW, Bae SH, et al. [Clinical features of patients with fulminant hepatitis A requiring emergency liver transplantation: comparison with acute liver failure due to other causes]. Korean J Hepatol. 2010; 16:19–28.

9. Rolando N, Wade J, Davalos M, Wendon J, Philpott-Howard J, Williams R. The systemic inflammatory response syndrome in acute liver failure. Hepatology. 2000; 32(4 Pt 1):734–739.

10. England JM, Down MC. Red-cell-volume distribution curves and the measurement of anisocytosis. Lancet. 1974; 1:701–703.

11. Oh J, Kang SM, Won H, Hong N, Kim SY, Park S, et al. Prognostic value of change in red cell distribution width 1 month after discharge in acute decompensated heart failure patients. Circ J. 2012; 76:109–116.

12. Oh HJ, Park JT, Kim JK, Yoo DE, Kim SJ, Han SH, et al. Red blood cell distribution width is an independent predictor of mortality in acute kidney injury patients treated with continuous renal replacement therapy. Nephrol Dial Transplant. 2012; 27:589–594.

13. Sadaka F, O'Brien J, Prakash S. Red cell distribution width and outcome in patients with septic shock. J Intensive Care Med. 2013; 28:307–313.

14. Lou Y, Wang M, Mao W. Clinical usefulness of measuring red blood cell distribution width in patients with hepatitis B. PLoS One. 2012; 7:e37644.

15. Corrao G, Aricò S. Independent and combined action of hepatitis C virus infection and alcohol consumption on the risk of symptomatic liver cirrhosis. Hepatology. 1998; 27:914–919.

16. Mullen KD. Review of the final report of the 1998 Working Party on definition, nomenclature and diagnosis of hepatic encephalopathy. Aliment Pharmacol Ther. 2007; 25:Suppl 1. 11–16.

17. Polson J, Lee WM. American Association for the Study of Liver Disease. AASLD position paper: the management of acute liver failure. Hepatology. 2005; 41:1179–1197.

19. Wiesner R, Edwards E, Freeman R, Harper A, Kim R, Kamath P, et al. Model for end-stage liver disease (MELD) and allocation of donor livers. Gastroenterology. 2003; 124:91–96.

20. Anand AC, Nightingale P, Neuberger JM. Early indicators of prognosis in fulminant hepatic failure: an assessment of the King's criteria. J Hepatol. 1997; 26:62–68.

21. NeSmith EG, Weinrich SP, Andrews JO, Medeiros RS, Hawkins ML, Weinrich M. Systemic inflammatory response syndrome score and race as predictors of length of stay in the intensive care unit. Am J Crit Care. 2009; 18:339–346.

22. Williams R. Classification, etiology, and considerations of outcome in acute liver failure. Semin Liver Dis. 1996; 16:343–348.

23. Ozsoylu S, Koçak N. Acute hepatic failure related to hepatitis A. Lancet. 1989; 1:901.
24. Kwon SY. Current status of liver diseases in Korea: hepatitis A. Korean J Hepatol. 2009; 15:Suppl 6. S7–S12.

25. Vaquero J, Polson J, Chung C, Helenowski I, Schiodt FV, Reisch J, et al. Infection and the progression of hepatic encephalopathy in acute liver failure. Gastroenterology. 2003; 125:755–764.

26. Craig DG, Reid TW, Martin KG, Davidson JS, Hayes PC, Simpson KJ. The systemic inflammatory response syndrome and sequential organ failure assessment scores are effective triage markers following paracetamol (acetaminophen) overdose. Aliment Pharmacol Ther. 2011; 34:219–228.

27. Kamath PS, Wiesner RH, Malinchoc M, Kremers W, Therneau TM, Kosberg CL, et al. A model to predict survival in patients with end-stage liver disease. Hepatology. 2001; 33:464–470.

28. Kamath PS, Kim WR. Advanced Liver Disease Study Group. The model for end-stage liver disease (MELD). Hepatology. 2007; 45:797–805.

29. Dhiman RK, Jain S, Maheshwari U, Bhalla A, Sharma N, Ahluwalia J, et al. Early indicators of prognosis in fulminant hepatic failure: an assessment of the Model for End-Stage Liver Disease (MELD) and King's College Hospital criteria. Liver Transpl. 2007; 13:814–821.

30. Katoonizadeh A, Decaestecker J, Wilmer A, Aerts R, Verslype C, Vansteenbergen W, et al. MELD score to predict outcome in adult patients with non-acetaminophen-induced acute liver failure. Liver Int. 2007; 27:329–334.

31. Perlstein TS, Weuve J, Pfeffer MA, Beckman JA. Red blood cell distribution width and mortality risk in a community-based prospective cohort. Arch Intern Med. 2009; 169:588–594.

33. Lednar WM, Lemon SM, Kirkpatrick JW, Redfield RR, Fields ML, Kelley PW. Frequency of illness associated with epidemic hepatitis A virus infections in adults. Am J Epidemiol. 1985; 122:226–233.





 PDF
PDF ePub
ePub Citation
Citation Print
Print






 XML Download
XML Download