Abstract
Purpose
Limited data are available on the role of percutaneous cardiopulmonary support (PCPS) for the treatment of acute myocardial infarction (AMI) patients with cardiogenic shock. We investigated the clinical outcomes and predictors of in-hospital mortality after PCPS in patients with AMI complicated by severe refractory cardiogenic shock.
Materials and Methods
From January 2004 to December 2011, we analyzed data from 96 consecutive AMI patients with cardiogenic shock assisted by a PCPS system. The primary outcome was in-hospital mortality. The predictors of in-hospital mortality were determined by a Cox proportional-hazards model.
Results
In-hospital mortality occurred in 51 (53.1%) patients and 58 (60.4%) patients were able to be weaned from PCPS. Cardiopulmonary resuscitation (CPR) was performed in 61 (63.5%) patients before PCPS initiation. On multivariate analysis, age ≥67 years [adjusted hazard ratio (HR), 4.74; 95% confidence interval (CI), 2.27-9.93; p<0.001], CPR (adjusted HR, 2.32; 95% CI, 1.11-4.85; p=0.03), lactate clearance for 48 hours <70% (adjusted HR, 2.50; 95% CI, 1.04-6.05; p=0.041), and unsuccessful revascularization (adjusted HR, 3.57; 95% CI, 1.85-6.90; p=0.002) were independent predictors of in-hospital mortality after PCPS in patients with AMI complicated by cardiogenic shock.
Although the mortality rate of patients with acute myocardial infarction (AMI) has much improved after the introduction of primary percutaneous coronary intervention (PCI), cardiogenic shock remains the leading cause of death in patients hospitalized with AMI.1,2 Therefore, additional hemodynamic support with primary PCI in AMI patients complicated by cardiogenic shock is very important. Mechanical circulatory support devices including intra-aortic balloon pumps (IABP), left ventricular assist devices (LVAD), and percutaneous cardiopulmonary support (PCPS) are used for maintaining hemodynamic support in cardiogenic shock.3 Several studies conducted in an emergent setting have indicated that PCPS might improve clinical outcomes in patients undergoing cardiogenic shock or arrest.4,5 However, limited data are available on the clinical outcomes of AMI patients requiring PCPS. Therefore, we investigated the clinical outcomes and predictors of in-hospital mortality after PCPS in patients with AMI complicated by intractable cardiogenic shock.
Between January 2004 and December 2011, PCPS was performed in 96 consecutive patients who had presented with an AMI (with or without ST-elevation) complicated by cardiogenic shock or witnessed in-hospital arrest. A patient was considered to be in cardiogenic shock if the patient had a systolic blood pressure of less than 90 mm Hg for more than 30 minutes after correction of hypovolemia, hypoxemia, and acidosis under maximal medical treatment including vasopressors. An arrest was presumed to be of cardiac etiology unless it was known or likely to have been caused by any other noncardiac cause. The following conditions were excluded in this study: age >80 years, previous severe neurologic damage, malignancy in the terminal stage, irreversible organ failure when no physiological benefit could be expected despite maximal therapy, and patients who previously signed "do-not-resuscitate" order. The study was approved by the Institutional Review Board.
A Capiox Emergency Bypass System (Capiox EBS™; Terumo Inc., Tokyo, Japan) was employed in all cases. This system comprises a portable controller with a back-up battery, a disposable bypass circuit integrated with a heparin-coated membrane oxygenator, and a centrifugal pump. The device was implanted by percutaneous cannulation using the Seldinger technique. Surgical cannulation using the cut-down method was also performed in difficult cases. Cannula sizes ranged from 14 to 21 Fr for the femoral artery and from 21 to 28 Fr for the femoral vein. In the event of distal limb ischemia after arterial cannulation, a catheter was inserted distal to the cannulation site for limb perfusion. Unfractionated heparin was continuously infused intravenously to maintain an activated clotting time between 180 and 220 sec. The initial flow rate of PCPS was set at 2.2 L/min/m2. The flow rate was adjusted to maintain a mean arterial pressure of 65 mm Hg. Echocardiography was performed daily to monitor the cardiac function. If the patient was hemodynamically stable and adequately oxygenated when the flow rate was 1 L/min/m2 for 4 hours, weaning off of the PCPS was considered. Successful weaning was defined as disconnection from PCPS without reinsertion or death within 24 hours. The termination of PCPS was considered with the consent of the family of the patient when there was intractable multi-organ failure or severe neurologic damage consistent with a vegetative state or brain death.
The primary outcome of the study was in-hospital mortality. To determine predictors of mortality, clinical data were obtained from medical record review; in-hospital data concerning age, gender, co-morbidities, and laboratory and procedural findings were collected. Successful revascularization was defined as residual stenosis <20% with the Thrombolysis in Myocardial Infarction (TIMI) 3 flow in all intervened lesions. Lactate level was measured every six hours from arterial or venous blood samples. The initial lactate level was defined as that obtained just before PCPS insertion. Lactate clearance for 48 hours (%) was defined according to following formula: lactate clearance for 48 hours (%)=[(highest lactate level for initial 6 hours-lowest lactate level for 24 to 48 hours)/highest lactate level for initial 6 hours]×100.
All values are presented as numbers with percentages for categorical variables and medians with interquartile ranges for continuous variables. Comparisons between continuous variables were made using a t-test or Mann-Whitney U test, as appropriate. Categorical data were analyzed using the chi-square test or Fisher's exact test, as appropriate. Event-free survival curves were estimated by the Kaplan-Meier method and compared using the log-rank test. The best discriminative values were obtained from a receiver operating characteristic (ROC) curve. Cox proportional hazard model was used to determine the predictors of in-hospital mortality on univariate and multivariate analysis. Covariates that were statistically significant on univariate analysis and those considered clinically relevant were included in the multivariate models. All tests were two-tailed and p<0.05 was considered statistically significant. SPSS version 20 (IBM, Armonk, NY, USA) was used for statistical analysis.
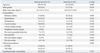
Between January 2004 and December 2011, ninety-six consecutive patients with AMI complicated by cardiogenic shock were enrolled in this study. The baseline characteristics and results of comparisons between in-hospital survivors (n=45) and non-survivors (n=51) are shown in Table 1. There were no significant differences in demographics or co-morbidities between the two groups, with the exception of age, where the non-survivors were older than the survivors (69 years vs. 63 years, p=0.009). Sixty-one (63.5%) patients underwent CPR just before or during PCPS placement. Although there was not a statistically significant difference in the number of patients who underwent CPR and those who did not, there was relatively more instances of CPR in the non-survivors [36/51 (70.6%) vs. 25/45 (55.6%), p=0.127]. The initial rhythm at the CPR was asystole in 13 (21.3%), pulseless electrical activity in 20 (32.8%), and ventricular tachycardia or fibrillation in 28 (45.9%). The median CPR duration was 27 (15-41) min, and there was a return of spontaneous circulation before PCPS was attempted in 20 (32.8%) patients. The median time from CPR onset to PCPS implantation was 36 (20-60) min (Supplementary Table 1 and 2, only online).
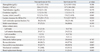
There were no significant differences in laboratory or procedural findings between the two groups, except for lactate level (Table 2). The non-survivors showed a higher initial lactate level (9.2 mmol/L vs. 5.9 mmol/L, p=0.041) and lower lactate clearance for 48 hours (47.5% vs. 75.2%, p=0.034) than the survivors. Vasopressor agents and mechanical ventilation were used in all but one and three patients, respectively. The IABP was used in 41 (42.7%) patients with PCPS. There were no significant differences in the concomitant use of IABP between the two groups.
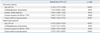
The in-hospital mortality was 53.1% (51/96) and 60.4% (58/96) of the patients were able to be weaned from PCPS (Table 3). A total of 25.4% (13/51) of the non-survivors were successfully weaned from PCPS but ultimately expired. Multiple organ failure with or without hemodynamic instability were the leading cause of death, occurring in each of 12 patients. The other causes of death were as follows: hypoxic brain damage in 11 patients, clinically suspected or culture-proven sepsis in 8 patients, uncontrolled bleeding in 6 patients, and extensive cerebral infarction in 2 patients. Revascularization was performed in 88 (91.7%) patients. Reasons for not receiving revascularization were diffuse or distal coronary artery disease not suitable for revascularization. Seven of 8 patients who did not undergo revascularization expired. There were no significant differences in in-hospital mortality between coronary artery bypass grafting (CABG) and PCI [CABG vs. PCI, 30.0% (3/10) vs. 52.6% (41/78), p=0.179] in patients undergoing revascularization. The Kaplan-Meier survival curves for the overall group and stratified by subgroup are provided in Fig. 1. We compared the clinical outcomes between patients with CPR and without CPR after PCPS, and there were no significant differences in the rate of in-hospital mortality, critical complications, and mortality during follow-up duration (Supplementary Table 3, Supplementary Fig. 1, only online).
On univariate analysis, age, lactate clearance for 48 hours, and unsuccessful revascularization were found to differ significantly between the non-survivors and the survivors (Table 4). Using a ROC curve analysis, the best discriminative values of age and lactate clearance for 48 hours to predict in-hospital mortality were determined to be 67 years (sensitivity 58.3%, specificity 75.0%, c-statistics=0.648) and 70% (sensitivity 60.0%, specificity 76.9%, c-statistics=0.689), respectively. Cox proportional-hazard models were adjusted with the following covariates: sex, diabetes mellitus, creatinine, ST elevation myocardial infarction, infarct-related artery, combined use of intra-aortic balloon pump, renal replacement therapy, and gastrointestinal bleeding. On multivariate analysis of non-survivors versus survivors, age ≥67 years [adjusted hazard ratio (HR), 4.74; 95% confidence interval (CI), 2.27-9.93; p<0.001], CPR (adjusted HR, 2.32; 95% CI, 1.11-4.85; p=0.03), lactate clearance for 48 hours <70% (adjusted HR, 2.50; 95% CI, 1.04-6.05; p=0.041), and unsuccessful revascularization (adjusted HR, 3.57; 95% CI, 1.85-6.90; p=0.002) were independent predictors of in-hospital mortality after PCPS in patients with AMI complicated by cardiogenic shock.
In patients who underwent PCPS without CPR, unsuccessful revascularization (adjusted HR, 7.92; 95% CI, 1.31-47.86; p=0.024) was an independent predictor of in-hospital mortality on multivariate analysis of non-survivors versus survivors. In patients who underwent PCPS during or after CPR, age ≥67 years (adjusted HR, 2.85; 95% CI, 1.13-7.17; p=0.026), unsuccessful revascularization (adjusted HR, 3.83; 95% CI, 1.59-9.22; p=0.003) and asystole or pulseless electrical activity as an initial rhythm (adjusted HR, 2.86; 95% CI, 1.06-7.68; p=0.038) were independent predictors of in-hospital mortality (Supplementary Table 4, only online).
The main findings of the present study are as follows: 1) the overall in-hospital mortality of patients with AMI complicated by cardiogenic shock requiring PCPS was 53.1%. 2) Older age (≥67 years), CPR, lower lactate clearance for 48 hours (<70%), and unsuccessful revascularization were significant predictors for in-hospital mortality.
The short-term mortality for cardiogenic shock complicating AMI was reported to be about 50% in recent studies.6,7 New mechanical assist devices have been utilized in an attempt to overcome the high mortality of cardiogenic shock. Even though IABP is the most commonly used device, the mortality of patients receiving IABP has not been found to be superior to non-IABP in recent studies, and IABP is often incapable of overcoming hemodynamic compromise in severe refractory cardiogenic shock.8,9 LVAD is documented to provide superior hemodynamic support than IABP for cardiogenic shock; however, LVAD is expensive and requires cardiac surgery for implantation, which limits its application as an emergent tool in patients with cardiogenic shock.10,11 On the other hand, PCPS is a pre-assembled, heparin-coated extracorporeal membrane oxygenation system that can be primed immediately and applied as a percutaneous approach in emergent situations.12
In the present study, the overall in-hospital mortality of AMI patients requiring PCPS was 53.1%. The mortality of our study is comparable, but slightly higher than that of the most recent studies on AMI, which reported in-hospital mortality rates ranging from 34% to 67%.13,14,15,16 The most likely explanation is patient selection. We included sixty-one (63.5%) patients complicated by cardiac arrest. Also, a return of spontaneous circulation before PCPS was attempted in twenty patients. The remaining forty-one patients were applied a combination of PCPS and intra-arrest PCI or CABG. The 30-day mortality in patients with combination of PCPS and intra-arrest PCI was reported as 71% in a recent study.17 In addition, we also included eight patients who did not achieve revascularization; seven of the 8 patients finally expired during hospitalization. Although PCPS provides stable hemodynamic conditions, perfusion of the infarction-related coronary arteries was still not enough.18 Revascularization is considered practical for preserving myocardial viability in patients with AMI as a bridge to recovery.19 In our study, the in-hospital mortality rate was 50.0% in patients who underwent revascularization and 44.4% in patients treated with successful revascularization. Furthermore, unsuccessful revascularization was a significant predictor of in-hospital mortality after adjusting for covariates on multivariate analysis. Accordingly, achievement of successful revascularization during PCPS may improve survival in AMI patients complicated by severe refractory cardiogenic shock.
Although lactate clearance is a clinically reliable tool for risk stratification in septic shock,20 limited data is currently available on the prognostic role of lactate clearance in cardiogenic shock complicating AMI. One pilot study advocated that 12-h lactate clearance <10% identifies a subset of patients at higher risk for death over the short- and long-term among patients with cardiogenic shock following AMI.21 In our study, lower lactate clearance for 48 hours (<70%) remained a significant predictor of in-hospital mortality after adjusting for covariates. Further studies performed in a larger cohort of AMI patients complicated with cardiogenic shock are needed to better elucidate the role of lactate clearance.
In patients who underwent PCPS during or after CPR, patients who presented with shockable rhythm such as ventricular fibrillation or ventricular tachycardia were more prevalent among survivors than those who presented with unshockable rhythm such as asystole or pulseless electrical activity. After adjusting covariates on multivariate analysis, initial rhythm was an additional predictor of in-hospital mortality. This result was consistent with our previous study, in which we proposed that defibrillation was an independent predictor of in-hospital survival during CPR assisted with PCPS.22
Our study had several limitations. First, each patient who could not be weaned from PCPS underwent LVAD and heart transplantation. We do not know exactly why the physicians would have decided on this course of action at that time, because of the limitations of a retrospective study. Second, hidden bias for PCPS initiation might remain, because the indication and timing of PCPS initiation was determined by the attending physicians in charge. Third, clinical outcomes of AMI patients supported with PCPS were not compared with medical therapy or other mechanical assist devices, such as IABP or LVAD. Therefore, we could not conclude the benefit of PCPS over medical therapy or other mechanical assist devices. As a randomized trial of strategy would be quite difficult to perform and the sample size of our study was larger than that of previous studies,13,14,15 our data could provide more assistive information about clinical outcomes of AMI shock treatment.
In conclusion, in spite of PCPS management, AMI patients complicated by severe refractory cardiogenic shock exhibited high mortality. Older age, CPR, lower lactate clearance for 48 hours, and unsuccessful revascularization were independent predictors of in-hospital mortality.
Figures and Tables
Fig. 1
Kaplan-Meier survival curve. (A) Kaplan-Meier survival curve for all patients. (B) Kaplan-Meier survival curve for age <67 years (solid line) versus age ≥67 years (dashed line). (C) Kaplan-Meier survival curve for lactate clearance for 48 hours ≥70% (solid line) versus lactate clearance for 48 hours <70% (dashed line). (D) Kaplan-Meier survival curve for successful revascularization (solid line) versus unsuccessful revascularization (dashed line).

References
1. Hochman JS, Sleeper LA, Webb JG, Sanborn TA, White HD, Talley JD, et al. SHOCK Investigators. Early revascularization in acute myocardial infarction complicated by cardiogenic shock. Should We Emergently Revascularize Occluded Coronaries for Cardiogenic Shock. N Engl J Med. 1999; 341:625–634.
2. Dauerman HL, Goldberg RJ, White K, Gore JM, Sadiq I, Gurfinkel E, et al. Revascularization, stenting, and outcomes of patients with acute myocardial infarction complicated by cardiogenic shock. Am J Cardiol. 2002; 90:838–842.

3. Cove ME, MacLaren G. Clinical review: mechanical circulatory support for cardiogenic shock complicating acute myocardial infarction. Crit Care. 2010; 14:235.

4. Chung SY, Sheu JJ, Lin YJ, Sun CK, Chang LT, Chen YL, et al. Outcome of patients with profound cardiogenic shock after cardiopulmonary resuscitation and prompt extracorporeal membrane oxygenation support. A single-center observational study. Circ J. 2012; 76:1385–1392.

5. Shin TG, Choi JH, Jo IJ, Sim MS, Song HG, Jeong YK, et al. Extracorporeal cardiopulmonary resuscitation in patients with inhospital cardiac arrest: a comparison with conventional cardiopulmonary resuscitation. Crit Care Med. 2011; 39:1–7.

6. Goldberg RJ, Spencer FA, Gore JM, Lessard D, Yarzebski J. Thirty-year trends (1975 to 2005) in the magnitude of, management of, and hospital death rates associated with cardiogenic shock in patients with acute myocardial infarction: a population-based perspective. Circulation. 2009; 119:1211–1219.

7. Babaev A, Frederick PD, Pasta DJ, Every N, Sichrovsky T, Hochman JS, et al. Trends in management and outcomes of patients with acute myocardial infarction complicated by cardiogenic shock. JAMA. 2005; 294:448–454.

8. Thiele H, Zeymer U, Neumann FJ, Ferenc M, Olbrich HG, Hausleiter J, et al. Intraaortic balloon support for myocardial infarction with cardiogenic shock. N Engl J Med. 2012; 367:1287–1296.

9. Sjauw KD, Engström AE, Vis MM, van der Schaaf RJ, Baan J Jr, Koch KT, et al. A systematic review and meta-analysis of intra-aortic balloon pump therapy in ST-elevation myocardial infarction: should we change the guidelines? Eur Heart J. 2009; 30:459–468.

10. Cheng JM, den Uil CA, Hoeks SE, van der Ent M, Jewbali LS, van Domburg RT, et al. Percutaneous left ventricular assist devices vs. intra-aortic balloon pump counterpulsation for treatment of cardiogenic shock: a meta-analysis of controlled trials. Eur Heart J. 2009; 30:2102–2108.

11. Kar B, Gregoric ID, Basra SS, Idelchik GM, Loyalka P. The percutaneous ventricular assist device in severe refractory cardiogenic shock. J Am Coll Cardiol. 2011; 57:688–696.

12. Sung K, Lee YT, Park PW, Park KH, Jun TG, Yang JH, et al. Improved survival after cardiac arrest using emergent autopriming percutaneous cardiopulmonary support. Ann Thorac Surg. 2006; 82:651–656.

13. Kim H, Lim SH, Hong J, Hong YS, Lee CJ, Jung JH, et al. Efficacy of veno-arterial extracorporeal membrane oxygenation in acute myocardial infarction with cardiogenic shock. Resuscitation. 2012; 83:971–975.

14. Morisawa D, Higuchi Y, Iwakura K, Okamura A, Date M, Ohmiya S, et al. Predictive factors for successful weaning from percutaneous cardiopulmonary support in patients with cardiogenic shock complicating acute myocardial infarction. J Cardiol. 2012; 60:350–354.

15. Tsao NW, Shih CM, Yeh JS, Kao YT, Hsieh MH, Ou KL, et al. Extracorporeal membrane oxygenation-assisted primary percutaneous coronary intervention may improve survival of patients with acute myocardial infarction complicated by profound cardiogenic shock. J Crit Care. 2012; 27:530.

16. Sakamoto S, Taniguchi N, Nakajima S, Takahashi A. Extracorporeal life support for cardiogenic shock or cardiac arrest due to acute coronary syndrome. Ann Thorac Surg. 2012; 94:1–7.

17. Kagawa E, Dote K, Kato M, Sasaki S, Nakano Y, Kajikawa M, et al. Should we emergently revascularize occluded coronaries for cardiac arrest?: rapid-response extracorporeal membrane oxygenation and intra-arrest percutaneous coronary intervention. Circulation. 2012; 126:1605–1613.

18. Rees MR, Browne T, Sivananthan UM, Whittaker S, Hick D, Verma SP, et al. Cardiac resuscitation with percutaneous cardiopulmonary support. Lancet. 1992; 340:513–514.

19. Wu MY, Tseng YH, Chang YS, Tsai FC, Lin PJ. Using extracorporeal membrane oxygenation to rescue acute myocardial infarction with cardiopulmonary collapse: the impact of early coronary revascularization. Resuscitation. 2013; 84:940–945.

20. Nguyen HB, Rivers EP, Knoblich BP, Jacobsen G, Muzzin A, Ressler JA, et al. Early lactate clearance is associated with improved outcome in severe sepsis and septic shock. Crit Care Med. 2004; 32:1637–1642.

Supplementary Material
Supplementary Fig. 1
Kaplan-Meier survival curve. Kaplan-Meier survival curve for PCPS without CPR (solid line) versus PCPS during or after CPR (dashed line). PCPS, percutaneous cardiopulmonary support; CPR, cardiopulmonary resuscitation.
Supplementary Table 1
Baseline Patient Characteristics between Patients Who Underwent PCPS during or after CPR and Those Who Underwent PCPS without CPR
Supplementary Table 2
Laboratory and Procedural Findings between Patients Who Underwent PCPS during or after CPR and Those Who Underwent PCPS without CPR




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download