Abstract
Presently, allergy diagnosis and therapy procedures are undergoing a transition phase in which allergen extracts are being step-by-step replaced by molecule-based products. The new developments will allow clinicians to obtain detailed information on sensitization patterns, more accurate interpretation of allergic symptoms, and thus improved patients' management. In this respect, recombinant technology has been applied to develop this new generation of molecule-based allergy products. The use of recombinant allergens allows full validation of identity, quantity, homogeneity, structure, aggregation, solubility, stability, IgE-binding and the biologic potency of the products. In contrast, such parameters are extremely difficult to assay and standardize for extract-based products. In addition to the possibility of bulk production of wild type molecules for diagnostic purposes, recombinant technology opened the possibility of developing safer and more efficacious products for allergy therapy. A number of molecule-based hypoallergenic preparations have already been successfully evaluated in clinical trials, bringing forward the next generation of allergy vaccines. In this contribution, we review the latest developments in allergen characterization, molecule-based allergy diagnosis, and the application of recombinant allergens in therapeutic setups. A comprehensive overview of clinical trials using recombinant allergens as well as synthetic peptides is presented.
Allergic diseases such as allergic rhinitis or hay fever, allergic asthma, food allergy, allergic skin inflammation, and anaphylaxis affect up to 25% of the population in industrialized countries and their incidence is continuously rising, particularly in children and young adults.1,2 The social and economic impact of allergic diseases is large, including costs of health care, lost work and school hours, and lower quality of life.3
Type I allergy is characterized by an overwhelming expansion of allergen-specific T helper 2 cells resulting in class switching of B cells to produce IgE antibodies specific to common environmental allergens originating from various sources, including pollen of grasses, weeds, or trees, spores of molds, foods, mites, cockroaches, and dander from pets and other domestic animals. The etiology of allergic diseases is multi-factorial: genetic susceptibility, route of exposure, the dose of the allergen, and in some instances, also the nature or structural characteristics of the allergen appear to influence the development of allergic disorders.1,2
Recombinant technology in the field of Allergology has brought tremendous advances in allergen characterization and vaccine development,4,5 as well as in the knowledge of immune mechanisms involved in allergic diseases.6,7 These advances have brought fresh opportunities for innovation in diagnostic and therapy of allergic diseases. Recent clinical trials with novel allergen preparations (synthetic peptides comprising T cell epitopes, recombinant allergens, and genetically engineered hypoallergens)7,8,9 and adjuvants derived from bacterial origin (monophosphoryl-lipid A or immunostimulatory DNA sequences)10 have delivered encouraging results. In addition, these new molecule-based vaccines offer the possibility of standardization in order to meet the highest pharmaceutical standards. Promising approaches to improve allergen-specific immunotherapy (SIT) include engineered hypoallergens (Ferreira), alternative delivery routes,11 and genetic immunization.12,13
Starting with the first attempts to understand allergic diseases at the beginning of the 20th century, allergen extracts have been developed for diagnostic and therapeutic purposes.14 Due to their biologic nature, extracts represent a heterogeneous mixture of proteins, glycoprotein, and polysaccharides from a given allergenic source, which makes standardization difficult, not to say practically impossible. The quality of an allergen extract is influenced by the production process but also by the source material, which may cause considerable variations. In fact, several studies have shown that the allergen content of extracts varies between different manufacturers as well as between batches.15,16 Standardization protocols to determine the potency of an extract start with skin prick tests on selected sensitized patients. According to the severity of the wheal-and-flare reaction an in-house reference is generated, which is used to validate the potency of subsequent batches of the allergenic product. For this purpose, serum pools are used in IgE-based inhibition assays. A good quality pool will consequently always contain enough IgE to determine the major allergenic compounds of an extract; however, differences in ratios of different allergens within a 840source are most likely masked by the assay. To improve extract quality and batch-to-batch consistency, the World Health Organization and the Allergen Standardization Sub-committee of the International Union of Immunological Societies developed in the 1980s reference preparations as reference standards for five allergenic preparations. However, these extracts, which were essentially identical to commercial products, never found broad acceptance among allergen manufacturers. Instead, the in-house references continued to be used.14 The United States Food and Drug Administration has currently standardized 19 allergenic extracts, including nine pollen, six venom, two mite, and two cat epidermal extracts, using ELISA with serum pools of allergic patients.17 Thus, manufacturers are required to demonstrate constancy and compliance of their products by using those standards.18
In the early 1990s, the European Union funded an initiative entitled "Development of Certified Reference Materials for Allergenic Products and Validation of Methods for their Quantification" also known under the acronym CREATE. The multi-disciplinary consortium included six allergen manufacturers, two biotech companies, three regulatory bodies, eleven clinicians, and six research institutions. The overall goal of the project was to generate reference standards based on the use of purified recombinant allergens and to develop and validate methods for the quantification of allergen content of extracts.14 As gold standards, purified natural allergens were used for evaluating the properties of recombinant proteins. In total, eight major inhalant allergens, among them birch Bet v 1, grass Phl p 1 and Phl p 5, mite Der p 1 and Der p 2, as well as Der f 1 and Der f 2, and olive Ole e 1 were selected for the project. All allergen preparations were physicochemically and immunologically characterized using diverse IgE binding assays (i.e., immunoblots, ELISA, or mediator release assays). Moreover, storage conditions for allergen preparations and long term stability were analyzed. Two of the investigated recombinant allergens, Bet v 1 and Phl p 5, qualified as candidates for allergen-standards.19 As a follow-up of this groundbreaking initiative to implement the use of recombinant allergens as certified reference standards, the European Directorate for the Quality of Medicines funded the Biological Standardization Program BSP090. The mission of this program is, amongst others, to elaborate European Pharmacopoeia Reference Standards and to develop test methods for biological.20 Based on the results of the CREATE project, recombinant Bet v 1 and the Phl p 5a isoform were selected as candidates to generate reference standards. Moreover, four ELISA systems from different manufacturers were included to quantify the respective allergens in both natural and recombinant preparations. The project was divided into three phases, a preliminary testing phase, an extended feasibility phase, and a phase confirming transferability of the methods. In brief, the results showed that both candidates proved suitable for the intended purpose and three out of four ELISA methods were positively evaluated.21
The full characterization of an allergen product beyond analyses of IgE potency seems mandatory to describe the full spectrum of molecular properties of a protein. Therefore, physicochemical analyses for the determination of identity, quantity, homogeneity, structural elements, aggregation, solubility, and stability can help to complement the picture obtained in ELISA or mediator release assays. As a gold standard to determine protein identity, a combination of mass spectrometric analyses with amino acid analysis has proven very efficacious. The latter method can further be used for protein quantification. To evaluate homogeneity and protein aggregation, size-exclusion chromatography combined with light scattering techniques and sodium dodecyl sulfate polyacrylamide gel electrophoresis has been frequently applied. To analyze folding and denaturation, spectroscopic techniques such as circular dichroism or Fourier transformed infrared spectroscopy provide valuable information. In addition, the immunologic parameters are usually assayed in ELISA and ELISA inhibition assays.22 Since allergens provide the raw material for many allergy-related products, the emphasis on careful allergen characterization has definitely contributed to increase the quality of diagnostic as well as therapeutic products.
Presently, allergy diagnosis is in a transition phase and a general process of rethinking the classical diagnostic procedures is ongoing (Fig. 1). Molecular or component-resolved allergy diagnosis is gaining importance and being increasingly applied in routine care.23 Below, recent developments in allergy diagnosis will be reviewed demonstrating that molecule-based approaches may offer more than simple IgE recognition profiles towards several dozens of allergenic molecules.
Typically, allergen sources contain multiple allergenic proteins, some of them being specific for a given source, while others show broad cross-reactivity. Moreover, different allergens are usually differently recognized by allergic patients and may also show different potencies in vivo. As demonstrated for the grass pollen allergens Phl p 4 and Phl p 13, their IgE recognition frequency was at 85% and 56%, respectively, though skin prick tests revealed that they exhibited a five- to nine-fold lower allergenic activity compared to Phl p 1, 2, or 5.24 In a study published in 2012 by Tripodi, et al.,25 a cohort of 200 allergic children with respiratory symptoms was screened using nine different pollen extracts. Children reactive to Phleum extract were further tested with a panel of eight different grass pollen allergens. Among 176 grass pollen allergic children, 39 different profiles of sensitization could be detected. This high heterogeneity strikingly demonstrates the limitations of extract-based diagnosis.
Weed pollen allergies caused by Asteraceae species ragweed and mugwort represent a serious health problem in late summer until autumn. Despite their botanical relationship, the major allergens of ragweed and mugwort have been identified as members of two distinct protein families. Amb a 1, the major ragweed allergen, belongs to the pectate lyase family, whereas the major allergen of mugwort, Art v 1, was classified as a two-domain glycoprotein. Ragweed Amb a 4 and mugwort Art v 6 represent the respective homologues of Art v 1 and Amb a 1, but both proteins have only been described as minor allergens within their source. Moreover, profilin and calcium-binding allergens have been identified within the two Asteracea species.26 In areas where both plants are endemic it seems virtually impossible to distinguish between molecular cross-reactivity and co-sensitization using allergen extracts for diagnosis. Co-sensitization by these two species seems a quite common phenomenon and cross-reactivity is mostly elicited by the panallergens profilin and Ca2+-binding proteins.27,28 A similar problem has been reported for venom allergies. In skin tests with Hymenoptera venom extracts, double positive results to extracts from bee and wasp venom are frequently observed, which could either be a result of cross-reactivity or true co-sensitization. Moreover, correct extract-based diagnosis of bee and wasp venom allergy is hampered by the fact that approximately 40% of venom allergic patients have specific IgE towards cross-reactive carbohydrate determinants (CCDs),29 which can further lead to false-positive diagnostic results due to sensitization to unrelated allergen sources (e.g., glycosylated food or pollen allergens).30 In this respect, species-specific marker allergens available as non-glycosylated recombinant proteins have been shown to be suitable tools for a more accurate venom allergy diagnosis.31
For the precise diagnosis of food allergies double-blind placebo-controlled food challenges still represent the gold standard; nevertheless, the method is costly, bears a high risk of inducing adverse side reactions, and is not always accessible. However, the use of extracts for the diagnosis of food allergies is problematic, as pointed out by several studies. For example, in a survey of the US population using skin prick test diagnosis with extracts, the sensitization rate to peanut was 8.6%, whereas the actual rate of clinical peanut allergy during the same time in the US population was between 0.5% and 1%.32,33 There is no clear explanation for this high discrepancy: it has been suggested that IgE cross-reactivity with pollen allergens (e.g., Bet v 1 or profilins) or CCDs may produce positive tests without causing peanut allergy.34
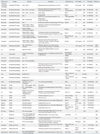
Because limitations of allergens extracts are evident, molecular allergy diagnosis has been developed as an alternative to investigate specific IgE binding to purified molecules (natural as well as recombinant). Beginning with the cloning of the first allergens in 1988, more than 2500 allergenic molecules have been identified so far.35,36 This huge number of allergens represents one of the pitfalls of molecular allergy diagnosis, and thus, the possibility to profile patients' IgE reactivities with a large number of allergens creates the need for algorithms to distill clinically important information out of the bulk of generated data (Fig. 1). Presently, manufacturers offer molecular allergy diagnosis in singleplex (i.e., Thermo Fisher ImmunoCAP, Siemens ImmuLite, and HyCor HyTec) or multiplex [Thermo Fisher Immuno-Solid phase Allergen Chip (ISAC)] formats; both systems have their advantages and limitations. Whereas in the singleplex format allergen extracts are frequently used to detect specific IgE, the current version of the microarray chip ISAC 112 offers the detection of 112 purified natural or recombinant allergens.37 On the other hand, unlike the singleplex assays, in the microarray format specific immunoglobulins are in excess relative to the amount of spotted allergen, which may render the assay biased towards the detection of high affinity antibody populations. Moreover, IgG interference with IgE binding might affect diagnostic outcomes.32 Nevertheless, one of the biggest advantages of molecular allergy diagnosis over extract-based methods is the ability to distinguish between genuine sensitization versus cross-reactivity. The performance of allergen microarrays to replace conventional extract-based allergy diagnosis has been evaluated in a series of studies summarized in Table 1.
Sensitivity and specificity are two crucial parameters in allergy diagnosis. The assay specificity is intimately connected with the selection of allergens included in the array system, which is reliant on the population to be investigated and on the quality of the protein preparations. A study on peanut allergy in a Spanish cohort population using the already revised ISAC 103 showed that the microarray had low diagnostic value because the peanut allergen Ara h 9, a non-specific lipid transfer protein, was not included in the array. There are reports on the cross-reactivity of Ara h 9 with peach Lipid Transfer Protein Pru p 3, which is considered a main sensitizer in the Mediterranean population.38 Of note, in the current version of the ISAC chip (112), Ara h 9 has been included in the allergen panel. Similarly, Wöhrl, et al.39 found that microarray diagnosis of mugwort allergy based on the use of recombinant Art v 1 was insufficient. A study by Gadermaier, et al.28 included a panel of five different mugwort pollen allergens (Art v 1, 3, 4, 5, and 6) and showed that. Besides Art v 1, sensitization to Art v 3 was frequently observed. In addition, cross-reactive allergens belonging to the polcalcin and profilin families showed considerable reactivity rates. In the same study, batches of natural as well as recombinant Amb a 1 were also tested. Whereas natural Amb a 1 was a good predictor of ragweed allergy, the recombinant protein failed to bind patients' serum IgE, demonstrating that the quality of the spotted allergens is decisive in assay performance.28
One of the biggest challenges of in vitro allergy diagnosis is to discriminate between sensitization and true allergy. Bet v 1-mediated birch pollen allergies are frequently associated with food allergies caused by cross-reactive IgE antibodies. Skin tests with fresh material usually show an excellent negative predictive value; however, positive prediction is low. Similarly, cross-reactivity of Bet v 1-induced IgE with various food sources is observable in vitro and often does not correlate with clinical allergy. In order to address this problem, several studies attempted to distinguish between true allergy and clinically irrelevant sensitization to Bet v 1-related food allergens. However, when using in vitro diagnosis, it is not yet possible to distinguish between sensitization and real allergy. Noteworthy, there was no significant difference in the assay performance of conventional sIgE tests or microarrays.40,41 In general, it has been demonstrated that in vitro diagnosis of clinical allergy is dependent on marker allergens. For instance, positive IgE-binding to kiwi Act d 1 was claimed as a predictive marker for genuine sensitization to kiwi fruit,42 and IgE binding to Ara h 2 has been suggested as a discriminator between tolerance and reactivity to peanuts.43 For the discrimination between latex allergy and sensitization, the latex allergens Hev b 1, 3, 5, 6, and 8, as well as a marker for CCD, were successfully tested in a molecule-based approach. Hev b 1, 3, 5, and 6 were identified as markers for latex allergy, while IgE binding to the latex profilin Hev b 8 was indicative for asymptotic sensitization.44
In summary, molecular allergy diagnosis is a valuable tool for a more accurate diagnosis. Especially for complex sensitization profiles, the identification of the disease-eliciting allergens is decisive for accurate prescription of therapeutic intervention. This question has actually been addressed by Sastre, et al.45 who determined the agreement coefficient for SIT before and after additional diagnosis with ISAC. In fact, there was agreement in only 46% of the cases after ISAC, indicating the additional value of molecular diagnosis for allergies.
Allergic diseases are complex immunologic disorders caused by various cellular and molecular mechanisms that lead to the pathophysiology of the allergic inflammation. SIT represents an intervention strategy capable of modifying the course of the disease even after its cessation. In parallel with developments in molecule-based allergy diagnosis to replace allergen extracts, attempts to generate SIT therapeutics based on highly purified and standardized molecules have emerged. A summary on clinical trials using molecule-based vaccine preparations is given in Table 2.
Recombinant technology not only allows the unlimited production of a particular protein, it also offers the possibility to fine-tune the intrinsic properties of the antigen. In other words, by using molecular approaches, IgE binding of wild-type allergens can be reduced, resulting in the generation of so-called hypoallergens, while the immunogenic properties of such molecules can be modulated. A problem arising with the use of recombinant allergens for therapy is the number of allergenic molecules within a given source. Some allergies (e.g., birch pollen or cat) are dominated by a single major allergen, whereas the majority of allergenic sources harbor more than one clinically relevant allergen.35 For instance Phleum pratense group 1, 2, 4, 5, and 6 allergens have been classified as major allergens, showing IgE reactivity with more than 50% of patients' sera; meanwhile, the pan allergens Phl p 12 (profilin) and Phl p 7 (calcium-binding protein) show IgE reactivities beyond 20%.46,47
For dust mite allergies, 24 allergen families have been identified to date (www.allergen.org). Moreover, in the case of house dust mite allergens a distinction between mite allergies in temperate and tropical climate zones has to be taken into consideration. In temperate climate zones, the Dermatophagoides species are dominant, whereas in the tropics Blomia species are the major source of allergens. The most dominant Dermatophagoides allergens are represented by group 1 and 2 allergens, accounting for 50% of IgE binding in mite extracts, whereas group 4, 5, 7, and 21 allergens were classified to be of medium potency. In Blomia, group 5 and 21 proteins are the disease dominating allergens, whereas the allergenicity of other mite allergens seems to be rather low. The highly cross-reactive tropomyosin shows a considerable degree of variation in IgE binding within different tested patients' cohorts.48 Such heterogeneous IgE recognition patterns represent a huge problem for the development of molecule based SIT reagents. Therefore, current developments for molecule-based immunotherapy focus on several aspects: 1) the identification of the most important disease-relevant components to diminish the number of allergens necessary for effective therapy; 2) the modification of these components to reduce IgE binding and consequently the possibility of side effects; and 3) optimization of the formulation and route of application of the allergens in order to maximize therapeutic efficacy. As mentioned above, the questions of how many allergens are necessary for an effective treatment and which one(s) to take could be readily answered for birch pollen or cat allergies. In fact, a randomized double-blind placebo-controlled clinical trial comparing the treatment efficacy of recombinant Bet v 1 with the natural protein or birch pollen extract in 134 birch pollen allergic patients showed a significant improvement in the patients' rhinoconjunctivitis scores paralleled with a reduction in skin reactivity. The improvement was independent of the intervention strategy, but not surprisingly, the extract-based treatment led to de novo induction of IgE towards the birch profilin Bet v 2 in three patients and to the elevation of Bet v 2-specific IgE antibodies in one subject. Of note, despite wild-type allergens were applied for therapy, the side effects of active and placebo-treated groups were similar.49 Presently, a tablet based on the use of recombinant Bet v 1.0101 is being developed, and in a phase IIb/III study, all three tested doses led to an improvement of the average adjusted symptom scores, defined as the primary endpoint of the study.50
For the treatment of grass pollen allergy, five dominant grass pollen allergens (Phl p 1, 2, Phl p 5 isoforms, and Phl p 6) were combined into a single vaccine, which was applied via subcutaneous injection. In a randomized double-blind placebo-controlled trial, 62 subjects received active treatment over a period of 1.5 years, which led to the induction of grass pollen specific IgG antibodies and suppression of specific IgE. Symptom and combined symptom-medication scores were significantly improved by the treatment, compared to placebo, and in a rhinitis quality of life questionnaire, significant differences between active and placebo treatment were observed in five out of seven categories. Reported side effects were mostly related to the injection sites; nevertheless, severe side effects including urticaria, dyspnea, and asthma exacerbation were reported. Reactions to placebo were most likely due to histamine included in the treatment.8 In a dose finding study performed with the same mixture of grass pollen allergens, four different maintenance doses ranging from 20 to 120 µg were tested following an up-dosing phase, which uniformly started with 0.156 µg/injection. As a primary end point of the study, the assessment of systemic reactions with a relationship to the intervention was defined. Recorded adverse systemic side effects grade I and II were rare and evenly distributed within the different groups. For determining treatment efficacy, conjunctival provocation tests were performed at which the 40 µg dose performed best. Furthermore, active treatment led to the induction of IgG antibodies and beneficial effects on late phase reactions, as determined by intra-cutaneous testing.51
To reduce the risk of treatment-induced side effects during SIT, recombinant hypoallergens showing diminished IgE binding properties have been developed and tested in clinical trials. The first hypoallergenic molecules to be tested were two recombinant fragments and a trimeric version of the major birch pollen allergen Bet v 1. In vitro, the fragments showed almost no IgE reactivity, whereas the T-cell activating properties, analyzed by re-stimulating Bet v 1-specific T cell clones, were retained.52 Similar findings were reported for the trimer.53 Both the fragments, as well as the trimer, were tested in a multicenter, placebo-controlled, double-blind, parallel-group, randomized trial on 124 birch pollen allergic patients. The intervention led to significant induction of Bet v 1-specific IgG antibodies, which were able to block allergen-induced mediator release on basophils.9 Active treatment showed trends to improve patients' wellbeing and nasal scores. However, the birch pollen season in one of the centers was very weak, thus by excluding this particular center, statistical significance in wellbeing was achieved in the trimer-treated group. In general, compared to the fragments, the trimer was more effective in ameliorating symptoms of birch pollen allergy. However, also side effects were most frequently associated with treatment with the trimer affecting 59.5% of patients compared to 37.8 and 30.6% for the fragments and placebo. Immediate side effects were generally mild and restricted to the injection site, but most side effects appeared several hours post injection and were very likely non-IgE mediated. Nevertheless, the high IgG titers induced by the hypoallergens plus the fact that de novo sensitizations, a common phenomenon of extract SIT, were not observed using recombinant hypoallergens encouraged further developments in this direction.54 The topic was therefore picked up by the group of Fiebig who developed a Bet v 1 hypoallergen by chemically altering the structure of the protein resulting in a fold variant of the major birch pollen allergen (Bet v 1-FV).55 Presently, the protein has been evaluated in clinical safety, efficacy, and dose finding studies. However, only the results of a rather small dose finding study have been published, stating that active treatment with Bet v 1-FV led to a significant increase of IgG1, but not IgG4, in all treatment groups, compared to placebo. Total symptom scores also decreased significantly in all actively treated groups. Side-effects were mostly associated with the two highest doses (160 and 320 µ), while the 80 µg dose showed even lower incidence of adverse effects than the lowest dose of 20 µg, indicating that the risk to benefit ratio with 80 µg Bet v 1-FV would be most favorable.56
More recently, the treatment of food allergies with SIT has been considered problematic since wild type allergen containing formulations might cause life-threatening side effects. Therefore, the hypoallergen concept provides an elegant alternative for the generation of safe vaccine candidates for food allergies. Along this line, two concepts have been developed, one for the major carp allergen Cyp s 1 and one for peach LTP Pru p 3. These approaches should ideally result in the generation of suitable hypoallergenic candidate molecules for food SIT. In both cases, the approach consists in the generation of several candidate molecules designed to be unable to adopt the WT-like structure, followed by in vitro and in vivo screening for most promising candidates, which should in turn be tested in Phase I/IIa, IIb randomized double-blind placebo-controlled trials. One focus of the ambitious project will be to understand the mechanisms of food SIT, which will guide future developments in this sector.57 Unlike carp or peach allergies, which are dominated by single disease eliciting allergens, allergic reactions to peanut are driven by multiple allergenic components, thus complicating the question of the optimal SIT vaccine composition. In a phase I study a combination of hypoallergenic variants of the peanut allergens Ara h 1, 2, and 3, which were encapsulated in heat/phenol killed, E. coli has been tested in five healthy subjects and 10 peanut allergic patients. To reduce IgE binding of peanut allergens, the allergens were dissected into linear epitopes, IgE binding was assessed and immunodominant epitopes were identified. Within the individual epitopes single amino acid positions were exchanged, abolishing IgE binding to the respective epitope.58,59 The proteins were thereafter expressed in E. coli and, after harvesting the cells carrying the respective allergens, were inactivated by heat/phenol treatment. The formulated product was applied rectal. All healthy individuals tolerated the product well; however, in the allergic group only four patients experienced no treatment-induced reaction. One patient had mild reactions; however, five experienced adverse reactions, which led to study dropout: three of these five subjects had more severe reactions including two cases of anaphylaxis. In general, the reactive subjects had higher IgE baseline, compared to non-reactive patients. Despite efficacy was not defined as study goal, skin prick test and basophil reactivity were significantly reduced by active treatment. Nevertheless, the high number of adverse side effects might have a negative impact on further developments in this direction.60
Besides using hypoallergens, several clinical studies have been conducted based on the use of peptides or fragments from major allergens of cat, grass pollen, ragweed, bee venom, and house dust mites (Table 2). Peptide immunotherapy is based on the rationale that IgE epitopes of most common allergens--food allergens represent an exception in this context--are conformational, thus by disruption of the allergen sequence into short fragments, IgE reactivity will be abrogated. During SIT, administered peptides will therefore not be able to induce IgE crosslinking, thus hindering the generation of an inflammatory milieu, which will lead to T cell tolerance in consequence (reviewed in61). In 2003, Fellrath, et al.62 published the results of a double-blind placebo-controlled phase I trial where long overlapping synthetic peptides covering the whole sequence of the major bee venom allergen phospholipase A2 were used to treat bee venom allergic patients. In general, the peptide therapy was well tolerated; especially, in the rapid up-dosing phase of 3.5 hours, no adverse reactions were reported. Thereafter, only mild side effects were observed in 2 of 9 actively treated patients. Peptide treatment induced specific IgG4 and T cell hypo-responsiveness in test subjects after an initial boost in T cell activation. Moreover, the cytokine profile was shifted towards IL-10 and IFN-γ production. Of note, a similar approach using overlapping peptides was recently tested for the birch allergen Bet v 1. In ELISA Bet v 1-derived peptides did not bind patients' IgE and also in human basophil mediator release assays mixtures of overlapping peptides were unable to activate degranulation. Moreover, in skin prick tests with birch pollen allergic donors, peptide combinations did not induce wheel and flare reactions.63 Still T cell reactivity and immunogenic properties in humans need to be researched. To address the problem of immunogenicity, Marth, et al.64 fused non IgE-reactive peptides of Bet v 1 to the hepatitis B surface protein, PreS, an approach that has also been tested for the major olive allergen Ole e 1.65 The hypothesis of this peptide carrier concept was to bypass the allergic IgE, as well as T cell reaction, and at the same time, to induce blocking antibodies against the WT allergens. Presently, the concept was tested in animal models and it will be interesting to know if this approach will also perform successfully in clinical trials.66
As an alternative to generate antibodies, peptides can also be used to precisely target allergen-specific T cells and to induce T cell tolerance during a therapeutic application. For many major allergens, T cell epitopes have been mapped, facilitating such an approach. In a randomized double-blind placebo controlled study on cat allergic individuals, the study subjects received either 6 nmol of a cat peptide formulation with 4-week intervals, 3 nmol in two-week intervals, or placebo. In total, seven different Fel d 1-derived peptides were mixed in an equimolar ratio. All peptides have previously been shown to be hypoallergenic.67 Rhinoconjunctivitis scores were assessed at 18 to 22 and 50 to 54 weeks post-treatment. After 1 year, the total rhino-conjunctivitis scores of the 6-nmol group were significantly improved over those of the 3-nmol and placebo groups. Moreover, active treatment with either concentration induced similar side effects as placebo, but none of the side effects were severe.68 Besides cat allergy, T cell peptide-based therapeutics for the treatment of allergies to grass, ragweed, and dust mites are under clinical investigations.
A cocktail of three T cell epitopes of the bee venom allergen phospholipase A2 were tested in five allergic patients and the clinical, as well as immunologic effects, were compared to conventional venom SIT. Peptide therapy did not induce side effects and after 2 months of therapy, all patients tolerated a challenge with 10 µg phospholipase A2, a dose which would correspond to the amount injected during a bee sting, without severe reactions. In fact, three patients did not show any reaction, while 2 patients developed mild symptoms. Of note, peptide therapy did not influence the antibody level per se, but the allergen challenge induced a significant increase in specific IgE, as well as IgG4 antibodies; nevertheless, the antibody ratio was in favor of IgG4. An increase in specific IgG4 was also seen in conventional SIT. In general, successful peptide SIT has been shown to have a suppressive effect on T cell activation.69
Figures and Tables
Fig. 1
The shift from extract-based to molecule-based allergy diagnosis. For molecule-based singleplex approaches, the number of tests to be performed can be very high. Allergen microarrays offer the advantage of testing a large panel of molecules in one single test.

ACKNOWLEDGEMENTS
The work of the authors has been supported by grants from the Austrian Science Fund (FWF L-688, W1213) and from the Christian Doppler Research Association.
References
1. Kay AB. Allergy and allergic diseases. Second of two parts. N Engl J Med. 2001; 344:109–113.
2. Kay AB. Allergy and allergic diseases. First of two parts. N Engl J Med. 2001; 344:30–37.
3. Masoli M, Fabian D, Holt S, Beasley R. Global Initiative for Asthma (GINA) Program. The global burden of asthma: executive summary of the GINA Dissemination Committee report. Allergy. 2004; 59:469–478.

4. Ferreira F, Briza P, Inführ D, Schmidt G, Wallner M, Wopfner N, et al. Modified recombinant allergens for safer immunotherapy. Inflamm Allergy Drug Targets. 2006; 5:5–14.

5. Valenta R, Niederberger V. Recombinant allergens for immunotherapy. J Allergy Clin Immunol. 2007; 119:826–830.

6. Akdis M, Akdis CA. Mechanisms of allergen-specific immunotherapy. J Allergy Clin Immunol. 2007; 119:780–791.

7. Larché M, Akdis CA, Valenta R. Immunological mechanisms of allergen-specific immunotherapy. Nat Rev Immunol. 2006; 6:761–771.

8. Jutel M, Jaeger L, Suck R, Meyer H, Fiebig H, Cromwell O. Allergen-specific immunotherapy with recombinant grass pollen allergens. J Allergy Clin Immunol. 2005; 116:608–613.

9. Niederberger V, Horak F, Vrtala S, Spitzauer S, Krauth MT, Valent P, et al. Vaccination with genetically engineered allergens prevents progression of allergic disease. Proc Natl Acad Sci U S A. 2004; 101:Suppl 2. 14677–14682.

10. Tulic MK, Fiset PO, Christodoulopoulos P, Vaillancourt P, Desrosiers M, Lavigne F, et al. Amb a 1-immunostimulatory oligodeoxynucleotide conjugate immunotherapy decreases the nasal inflammatory response. J Allergy Clin Immunol. 2004; 113:235–241.

11. Moingeon P, Batard T, Fadel R, Frati F, Sieber J, Van Overtvelt L. Immune mechanisms of allergen-specific sublingual immunotherapy. Allergy. 2006; 61:151–165.

12. Weiss R, Hammerl P, Hartl A, Hochreiter R, Leitner WW, Scheiblhofer S, et al. Design of protective and therapeutic DNA vaccines for the treatment of allergic diseases. Curr Drug Targets Inflamm Allergy. 2005; 4:585–597.

13. Weiss R, Scheiblhofer S, Gabler M, Ferreira F, Leitner WW, Thalhamer J. Is genetic vaccination against allergy possible? Int Arch Allergy Immunol. 2006; 139:332–345.

14. van Ree R. CREATE Partnership. The CREATE project: EU support for the improvement of allergen standardization in Europe. Allergy. 2004; 59:571–574.

15. Akkerdaas JH, Wensing M, Knulst AC, Krebitz M, Breiteneder H, de Vries S, et al. How accurate and safe is the diagnosis of hazelnut allergy by means of commercial skin prick test reagents? Int Arch Allergy Immunol. 2003; 132:132–140.

16. Curin M, Reininger R, Swoboda I, Focke M, Valenta R, Spitzauer S. Skin prick test extracts for dog allergy diagnosis show considerable variations regarding the content of major and minor dog allergens. Int Arch Allergy Immunol. 2011; 154:258–263.

17. U.S. Food and Drug Administration. Standardized Allergenic Extracts. USA: U.S. Food and Drug Administration;2009. Available from: http://www.fda.gov/BiologicsBloodVaccines/Allergenics/ucm391514.htm.
18. Jeong KY, Hong CS, Lee JS, Park JW. Optimization of allergen standardization. Yonsei Med J. 2011; 52:393–400.

19. van Ree R, Chapman MD, Ferreira F, Vieths S, Bryan D, Cromwell O, et al. The CREATE project: development of certified reference materials for allergenic products and validation of methods for their quantification. Allergy. 2008; 63:310–326.

20. EDQM. EDQM Annual Report 2011. France: EDQM;2011. Available from: http://www.edqm.eu/en/EDQM-Downloads-527.html.
21. Vieths S, Barber D, Chapman M, Costanzo A, Daas A, Fiebig H, et al. Establishment of recombinant major allergens Bet v 1 and Phl p 5a as Ph. Eur. reference standards and validation of ELISA methods for their measurement. Results from feasibility studies. Pharmeur Bio Sci Notes. 2012; 2012:118–134.
22. Himly M, Nony E, Chabre H, Van Overtvelt L, Neubauer A, van Ree R, et al. Standardization of allergen products: 1. Detailed characterization of GMP-produced recombinant Bet v 1.0101 as biological reference preparation. Allergy. 2009; 64:1038–1045.

23. Canonica GW, Ansotegui IJ, Pawankar R, Schmid-Grendelmeier P, van Hage M, Baena-Cagnani CE, et al. A WAO-ARIA-GA2LEN consensus document on molecular-based allergy diagnostics. World Allergy Organ J. 2013; 6:17.

24. Westritschnig K, Horak F, Swoboda I, Balic N, Spitzauer S, Kundi M, et al. Different allergenic activity of grass pollen allergens revealed by skin testing. Eur J Clin Invest. 2008; 38:260–267.

25. Tripodi S, Frediani T, Lucarelli S, Macrì F, Pingitore G, Di Rienzo Businco A, et al. Molecular profiles of IgE to Phleum pratense in children with grass pollen allergy: implications for specific immunotherapy. J Allergy Clin Immunol. 2012; 129:834–839.

26. Gadermaier G, Hauser M, Ferreira F. Allergens of weed pollen: an overview on recombinant and natural molecules. Methods. 2014; 66:55–66.

27. Asero R, Wopfner N, Gruber P, Gadermaier G, Ferreira F. Artemisia and Ambrosia hypersensitivity: co-sensitization or co-recognition? Clin Exp Allergy. 2006; 36:658–665.

28. Gadermaier G, Wopfner N, Wallner M, Egger M, Didierlaurent A, Regl G, et al. Array-based profiling of ragweed and mugwort pollen allergens. Allergy. 2008; 63:1543–1549.

29. Müller UR, Johansen N, Petersen AB, Fromberg-Nielsen J, Haeberli G. Hymenoptera venom allergy: analysis of double positivity to honey bee and Vespula venom by estimation of IgE antibodies to species-specific major allergens Api m1 and Ves v5. Allergy. 2009; 64:543–548.

30. Hemmer W, Focke M, Kolarich D, Wilson IB, Altmann F, Wöhrl S, et al. Antibody binding to venom carbohydrates is a frequent cause for double positivity to honeybee and yellow jacket venom in patients with stinging-insect allergy. J Allergy Clin Immunol. 2001; 108:1045–1052.

31. Müller U, Schmid-Grendelmeier P, Hausmann O, Helbling A. IgE to recombinant allergens Api m 1, Ves v 1, and Ves v 5 distinguish double sensitization from crossreaction in venom allergy. Allergy. 2012; 67:1069–1073.

32. Shreffler WG. Microarrayed recombinant allergens for diagnostic testing. J Allergy Clin Immunol. 2011; 127:843–849.

33. Arbes SJ Jr, Gergen PJ, Elliott L, Zeldin DC. Prevalences of positive skin test responses to 10 common allergens in the US population: results from the third National Health and Nutrition Examination Survey. J Allergy Clin Immunol. 2005; 116:377–383.

34. Codreanu F, Collignon O, Roitel O, Thouvenot B, Sauvage C, Vilain AC, et al. A novel immunoassay using recombinant allergens simplifies peanut allergy diagnosis. Int Arch Allergy Immunol. 2011; 154:216–226.

35. Cromwell O, Häfner D, Nandy A. Recombinant allergens for specific immunotherapy. J Allergy Clin Immunol. 2011; 127:865–872.

36. Allergome [Internet]. Italy: Allergome;2013. Available from: http://www.allergome.org.
37. Termo Fisher Scientific. ImmunoCAP Allergen list [Internet]. Sweden: Termo Fisher Scientific;2013. Available from: http://www.phadia.com/en/Products/Allergy-testing-products/ImmunoCAP-Allergen-Information/.
38. Javaloyes G, Goikoetxea MJ, García Núñez I, Sanz ML, Blanca M, Scheurer S, et al. Performance of different in vitro techniques in the molecular diagnosis of peanut allergy. J Investig Allergol Clin Immunol. 2012; 22:508–513.
39. Wöhrl S, Vigl K, Zehetmayer S, Hiller R, Jarisch R, Prinz M, et al. The performance of a component-based allergen-microarray in clinical practice. Allergy. 2006; 61:633–639.

40. Ebo DG, Bridts CH, Verweij MM, De Knop KJ, Hagendorens MM, De Clerck LS, et al. Sensitization profiles in birch pollen-allergic patients with and without oral allergy syndrome to apple: lessons from multiplexed component-resolved allergy diagnosis. Clin Exp Allergy. 2010; 40:339–347.

41. Villalta D, Asero R. Is the detection of IgE to multiple Bet v 1-homologous food allergens by means of allergen microarray clinically useful? J Allergy Clin Immunol. 2010; 125:1158–1161.

42. Bublin M, Dennstedt S, Buchegger M, Antonietta Ciardiello M, Bernardi ML, Tuppo L, et al. The performance of a component-based allergen microarray for the diagnosis of kiwifruit allergy. Clin Exp Allergy. 2011; 41:129–136.

43. Nicolaou N, Poorafshar M, Murray C, Simpson A, Winell H, Kerry G, et al. Allergy or tolerance in children sensitized to peanut: prevalence and differentiation using component-resolved diagnostics. J Allergy Clin Immunol. 2010; 125:191–197.

44. Ebo DG, Hagendorens MM, De Knop KJ, Verweij MM, Bridts CH, De Clerck LS, et al. Component-resolved diagnosis from latex allergy by microarray. Clin Exp Allergy. 2010; 40:348–358.

45. Sastre J, Landivar ME, Ruiz-García M, Andregnette-Rosigno MV, Mahillo I. How molecular diagnosis can change allergen-specific immunotherapy prescription in a complex pollen area. Allergy. 2012; 67:709–711.

46. Andersson K, Lidholm J. Characteristics and immunobiology of grass pollen allergens. Int Arch Allergy Immunol. 2003; 130:87–107.

47. Sekerkova A, Polackova M, Striz I. Detection of Phl p 1, Phl p 5, Phl p 7 and Phl p 12 specific IgE antibodies in the sera of children and adult patients allergic to Phleum pollen. Allergol Int. 2012; 61:339–346.

48. Thomas WR, Hales BJ, Smith WA. House dust mite allergens in asthma and allergy. Trends Mol Med. 2010; 16:321–328.

49. Pauli G, Larsen TH, Rak S, Horak F, Pastorello E, Valenta R, et al. Efficacy of recombinant birch pollen vaccine for the treatment of birch-allergic rhinoconjunctivitis. J Allergy Clin Immunol. 2008; 122:951–960.

50. Larenas-Linnemann D. Oralair Birch, a recombinant major birch pollen allergen tablet for sublingual immunotherapy of allergic rhinitis caused by birch pollen. Curr Opin Investig Drugs. 2010; 11:586–596.
51. Klimek L, Schendzielorz P, Pinol R, Pfaar O. Specific subcutaneous immunotherapy with recombinant grass pollen allergens: first randomized dose-ranging safety study. Clin Exp Allergy. 2012; 42:936–945.

52. Vrtala S, Hirtenlehner K, Vangelista L, Pastore A, Eichler HG, Sperr WR, et al. Conversion of the major birch pollen allergen, Bet v 1, into two nonanaphylactic T cell epitope-containing fragments: candidates for a novel form of specific immunotherapy. J Clin Invest. 1997; 99:1673–1681.

53. Vrtala S, Hirtenlehner K, Susani M, Akdis M, Kussebi F, Akdis CA, et al. Genetic engineering of a hypoallergenic trimer of the major birch pollen allergen Bet v 1. FASEB J. 2001; 15:2045–2047.

54. Purohit A, Niederberger V, Kronqvist M, Horak F, Grönneberg R, Suck R, et al. Clinical effects of immunotherapy with genetically modified recombinant birch pollen Bet v 1 derivatives. Clin Exp Allergy. 2008; 38:1514–1525.

55. Kahlert H, Suck R, Weber B, Nandy A, Wald M, Keller W, et al. Characterization of a hypoallergenic recombinant Bet v 1 variant as a candidate for allergen-specific immunotherapy. Int Arch Allergy Immunol. 2008; 145:193–206.

56. Meyer W, Narkus A, Salapatek AM, Häfner D. Double-blind, placebo-controlled, dose-ranging study of new recombinant hypoallergenic Bet v 1 in an environmental exposure chamber. Allergy. 2013; 68:724–731.

57. Zuidmeer-Jongejan L, Fernandez-Rivas M, Poulsen LK, Neubauer A, Asturias J, Blom L, et al. FAST: towards safe and effective subcutaneous immunotherapy of persistent life-threatening food allergies. Clin Transl Allergy. 2012; 2:5.

58. Stanley JS, King N, Burks AW, Huang SK, Sampson H, Cockrell G, et al. Identification and mutational analysis of the immunodominant IgE binding epitopes of the major peanut allergen Ara h 2. Arch Biochem Biophys. 1997; 342:244–253.

59. Burks AW, Shin D, Cockrell G, Stanley JS, Helm RM, Bannon GA. Mapping and mutational analysis of the IgE-binding epitopes on Ara h 1, a legume vicilin protein and a major allergen in peanut hypersensitivity. Eur J Biochem. 1997; 245:334–339.

60. Wood RA, Sicherer SH, Burks AW, Grishin A, Henning AK, Lindblad R, et al. A phase 1 study of heat/phenol-killed, E. coli-encapsulated, recombinant modified peanut proteins Ara h 1, Ara h 2, and Ara h 3 (EMP-123) for the treatment of peanut allergy. Allergy. 2013; 68:803–808.

62. Fellrath JM, Kettner A, Dufour N, Frigerio C, Schneeberger D, Leimgruber A, et al. Allergen-specific T-cell tolerance induction with allergen-derived long synthetic peptides: results of a phase I trial. J Allergy Clin Immunol. 2003; 111:854–861.

63. Pellaton C, Perrin Y, Boudousquié C, Barbier N, Wassenberg J, Corradin G, et al. Novel birch pollen specific immunotherapy formulation based on contiguous overlapping peptides. Clin Transl Allergy. 2013; 3:17.

64. Marth K, Breyer I, Focke-Tejkl M, Blatt K, Shamji MH, Layhadi J, et al. A nonallergenic birch pollen allergy vaccine consisting of hepatitis PreS-fused Bet v 1 peptides focuses blocking IgG toward IgE epitopes and shifts immune responses to a tolerogenic and Th1 phenotype. J Immunol. 2013; 190:3068–3078.

65. Twaroch TE, Focke M, Civaj V, Weber M, Balic N, Mari A, et al. Carrier-bound, nonallergenic Ole e 1 peptides for vaccination against olive pollen allergy. J Allergy Clin Immunol. 2011; 128:178–184.

66. Focke M, Swoboda I, Marth K, Valenta R. Developments in allergen-specific immunotherapy: from allergen extracts to allergy vaccines bypassing allergen-specific immunoglobulin E and T cell reactivity. Clin Exp Allergy. 2010; 40:385–397.

67. Worm M, Lee HH, Kleine-Tebbe J, Hafner RP, Laidler P, Healey D, et al. Development and preliminary clinical evaluation of a peptide immunotherapy vaccine for cat allergy. J Allergy Clin Immunol. 2011; 127:89–97.

68. Patel D, Couroux P, Hickey P, Salapatek AM, Laidler P, Larché M, et al. Fel d 1-derived peptide antigen desensitization shows a persistent treatment effect 1 year after the start of dosing: a randomized, placebo-controlled study. J Allergy Clin Immunol. 2013; 131:103–109.
69. Müller U, Akdis CA, Fricker M, Akdis M, Blesken T, Bettens F, et al. Successful immunotherapy with T-cell epitope peptides of bee venom phospholipase A2 induces specific T-cell anergy in patients allergic to bee venom. J Allergy Clin Immunol. 1998; 101(6 Pt 1):747–754.

70. Hauser M, Asam C, Himly M, Palazzo P, Voltolini S, Montanari C, et al. Bet v 1-like pollen allergens of multiple Fagales species can sensitize atopic individuals. Clin Exp Allergy. 2011; 41:1804–1814.

71. Jahn-Schmid B, Harwanegg C, Hiller R, Bohle B, Ebner C, Scheiner O, et al. Allergen microarray: comparison of microarray using recombinant allergens with conventional diagnostic methods to detect allergen-specific serum immunoglobulin E. Clin Exp Allergy. 2003; 33:1443–1449.

72. Cabrera-Freitag P, Goikoetxea MJ, Beorlegui C, Gamboa P, Gastaminza G, Fernández-Benítez M, et al. Can component-based microarray replace fluorescent enzimoimmunoassay in the diagnosis of grass and cypress pollen allergy? Clin Exp Allergy. 2011; 41:1440–1446.

73. Bronnert M, Mancini J, Birnbaum J, Agabriel C, Liabeuf V, Porri F, et al. Component-resolved diagnosis with commercially available D. pteronyssinus Der p 1, Der p 2 and Der p 10: relevant markers for house dust mite allergy. Clin Exp Allergy. 2012; 42:1406–1415.

74. Ott H, Baron JM, Heise R, Ocklenburg C, Stanzel S, Merk HF, et al. Clinical usefulness of microarray-based IgE detection in children with suspected food allergy. Allergy. 2008; 63:1521–1528.

75. D'Urbano LE, Pellegrino K, Artesani MC, Donnanno S, Luciano R, Riccardi C, et al. Performance of a component-based allergen-microarray in the diagnosis of cow's milk and hen's egg allergy. Clin Exp Allergy. 2010; 40:1561–1570.
76. Alessandri C, Zennaro D, Scala E, Ferrara R, Bernardi ML, Santoro M, et al. Ovomucoid (Gal d 1) specific IgE detected by microarray system predict tolerability to boiled hen's egg and an increased risk to progress to multiple environmental allergen sensitisation. Clin Exp Allergy. 2012; 42:441–450.

77. Hofmann SC, Fischer J, Eriksson C, Bengtsson Gref O, Biedermann T, Jakob T. IgE detection to α/β/γ-gliadin and its clinical relevance in wheat-dependent exercise-induced anaphylaxis. Allergy. 2012; 67:1457–1460.

78. Berneder M, Bublin M, Hoffmann-Sommergruber K, Hawranek T, Lang R. Allergen chip diagnosis for soy-allergic patients: Gly m 4 as a marker for severe food-allergic reactions to soy. Int Arch Allergy Immunol. 2013; 161:229–233.

79. Schuler S, Ferrari G, Schmid-Grendelmeier P, Harr T. Microarray-based component-resolved diagnosis of latex allergy: isolated IgE-mediated sensitization to latexprofilin Hev b8 may act as confounder. Clin Transl Allergy. 2013; 3:11.

80. Ott H, Schröder C, Raulf-Heimsoth M, Mahler V, Ocklenburg C, Merk HF, et al. Microarrays of recombinant Hevea brasiliensis proteins: a novel tool for the component-resolved diagnosis of natural rubber latex allergy. J Investig Allergol Clin Immunol. 2010; 20:129–138.
81. Winther L, Poulsen LK, Robin B, Melac M, Malling HJ. Safety and tolerability of recombinant Bet v 1 (rBet v 1) tablets in sublingual immunotherapy (SLIT) [abstract]. J Allergy Clin Immunol. 2009; 123:Suppl. S215.
82. Reisinger J, Horak F, Pauli G, van Hage M, Cromwell O, König F, et al. Allergen-specific nasal IgG antibodies induced by vaccination with genetically modified allergens are associated with reduced nasal allergen sensitivity. J Allergy Clin Immunol. 2005; 116:347–354.

83. Niederberger V, Reisinger J, Valent P, Krauth MT, Pauli G, van Hage M, et al. Vaccination with genetically modified birch pollen allergens: immune and clinical effects on oral allergy syndrome. J Allergy Clin Immunol. 2007; 119:1013–1016.

84. Pree I, Shamji MH, Kimber I, Valenta R, Durham SR, Niederberger V. Inhibition of CD23-dependent facilitated allergen binding to B cells following vaccination with genetically modified hypoallergenic Bet v 1 molecules. Clin Exp Allergy. 2010; 40:1346–1352.

85. Pauli G, Purohit A, Oster JP, De Blay F, Vrtala S, Niederberger V, et al. Comparison of genetically engineered hypoallergenic rBet v 1 derivatives with rBet v 1 wild-type by skin prick and intradermal testing: results obtained in a French population. Clin Exp Allergy. 2000; 30:1076–1084.

86. Senti G, Crameri R, Kuster D, Johansen P, Martinez-Gomez JM, Graf N, et al. Intralymphatic immunotherapy for cat allergy induces tolerance after only 3 injections. J Allergy Clin Immunol. 2012; 129:1290–1296.

87. Zhang K, Zhu D, Kepley C, Terada T, Saxon A. Chimeric human fcgamma-allergen fusion proteins in the prevention of allergy. Immunol Allergy Clin North Am. 2007; 27:93–103.
88. Maguire P, Nicodemus C, Robinson D, Aaronson D, Umetsu DT. The safety and efficacy of ALLERVAX CAT in cat allergic patients. Clin Immunol. 1999; 93:222–231.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download