Abstract
Acute vanishing bile duct syndrome, a rare but rapidly progressive destruction of the intrahepatic bile ducts with unknown pathogenesis, is most often a drug- or toxin-related. Toxic epidermal necrolysis is a serious dermatologic condition and a potentially life threatening disease, which is drug or infection induced. Ibuprofen associated acute vanishing bile duct syndrome and toxic epidermal necrolysis have not been reported previously in infants. We report a 7-month-old infant with ibuprofen associated toxic epidermal necrolysis, followed by severe and rapidly progressive vanishing bile duct syndrome. She recovered totally with supportive care.
Vanishing bile duct syndrome (VBDS) is a rare cause of progressive destruction and disappearance of the intrahepatic bile ductsm ultimately resulting in cholestasis. The acute form is often drug-related.1,2 The commonly implicated drugs are anticonvulsant and antidepressants.1,2 The pathogenesis of VBDS is unknown, and both immune-mediated and idiosyncrastic metabolic mechanisms have been proposed1,3 Toxic epidermal necrolysis (TEN) is an uncommon but severe dermatologic condition that is characterized by erythema with bullous and eroded lesions of skin and mucous membranes, often drug- or infection-induced.4,5 TEN is well recognized immune complex-mediated hypersensitivity reactions,1,4,5 suggesting shared immune mechanisms in the pathogenesis of both TEN and VBDS.1,4,5,6,7 VBDS with TEN is extremely rare and the prognosis is unknown, especially in children. To our best knowledge, ibuprofen-associated VBDS and TEN in infant have never been reported previously. We report herein a 7-month-old infant with ibuprofen-associated TEN, followed by severe and rapidly progressive VBDS.
A 7-month-old girl was hospitalized with complaints of fever and erythematous rashes on body. Two days earlier, she had fever and received ibuprofen at conventional pediatric doses (maximum of 30 mg/kg/day). She had no history of allergic disease. Three months ago, she had been treated with one-dose of ibuprofen (10 mg/kg) for sporadic fever. There was no family history of atopic disease, immunodeficiency or hepatobiliary disease. On admission, physical examination revealed macular erythematous eruption on her face, trunk, and arms, which progressed within 8 hours into the bullae around the arms, and involved the face and trunk. Skin lesion covered more than 30 percent of her body surface area. There were no lesions in the lips or in the eyes. A diagnosis of toxic epidermal necrolysis was made.
The initial peripheral white cell count was 5910/mm3 with 69.7% neutrophils, 20.3% lymphocytes, and 3% eosinophils. Hemoglobin level was 12.2 g/dL, platelets count 250000/mm3, erythrocyte sedimentation rate of 120 mm/h, and C-reactive protein 9 mg/dL. Aspartate aminotransferase (AST) level was 716 IU/L, alanine aminotransferase (ALT) 523 IU/L, alkaline phosphatase (ALP) 500 IU/L, total bilirubin (TB) 0.52 mg/dL and direct bilirubin (DB) 0.15 mg/dL. Serological test for hepatitis A, B, and C, Epstein Barr virus, parvovirus B19, herpes virus, adenovirus, cytomegalovirus, human immunodeficiency virus, leptospira, and mycoplasma were negative.
At hospital day 6, fever and cutaneous lesion resolved after supportive care but cholestatic picture was presented. AST level was 879 IU/L, ALT 723 IU/L, ALP 890 IU/L, TB 8.5 mg/dL, DB 5.7 mg/dL, gamma-glutamyl transferase 270 IU/L, and total cholesterol 760 mg/dL. The following laboratory findings were normal: amylase, lipase, prothrombin and partial thromboplastin time, α1-antitrypsin, immunoglobulins, complement levels, antinuclear antibody, anti-DNA antibody, anti-smooth muscle antibody, antimitochondrial antibody, anti-liver/kidney microsomal, anti-cytosol antibody, and antineutrophil cytoplasmic antibody. Abdominal ultrasound showed that the liver had homogeneous texture with normal bile ducts and gallbladder. Ursodeoxycholic acid treatment (15 mg/kg per day) was administered.
At hospital day 20, skin lesion recovered, but the TB and DB levels were still high (TB 9.5 mg/dL, DB 7.7 mg/dL). Liver biopsy was performed 20 days after admission. It showed lymphocyte infiltrations, marked degeneration of the interlobular bile duct epithelium, and the destructive narrowing of the ductules in the portal tracts and no intralobar bile ducts in at least 10 portal areas on H&E stain, suggesting VBDS (Fig. 1). Neither an organism nor viral cytopathic effect was identified. There was no significant hepatocellular damage and no evidence of sclerosing cholangitis or autoimmune hepatitis.
The diagnosis of VBDS associated with TEN was made. During a follow-up period of 3 months, clinical symptoms and biochemical data had shown tendency for resolution. The ursodeoxycholic acid treatment was discontinued. During the next three years, she has a normal physical status with normal liver synthetic functions.
Drug associated VBDS with TEN is extremely rare in children. In the present case, there was a close temporal relationship between ibuprofen administration and the appearance of skin lesion and liver dysfunction. Moreover, other drugs were not taken, and other potential causes were excluded on the basis of appropriate serological and histological results. Therefore, it may reasonably be ascribed to ibuprofen associated VBDS with TEN. To our knowledge, this patient is the first infant reported to have ibuprofen-associated VBDS with TEN.
Ibuprofen is a member of propionic acid class of nonsteroidal anti-inflammatory drug (NSAID), and appears to have low incidence of liver damage.1,2 The pattern of liver injury is typically hepatocellular but can be mixed or cholestatic.8 Most cases are mild-to-moderate in severity and rapidly reversible on stopping ibuprofen. Several reports have been published on severe protracted cholestasis and acute liver failure.9,10 The mechanism of drug induced VBSD is not fully understood, however, may be multi-factorial, and toxic, idiosyncratic and immune causes have been suggested.1,2,9,10 In our present case, first exposure to ibuprofen might have sensitized her. Liver injury might be due to an immune-allergic mechanism because of the association with TEN, which is immune complex medicated with hypersensitivity reactions.4
Drug-induced VBDS and skin injury are still unknown particularly in children. A literature search revealed six pediatric case reports with drug-associated VBDS with skin lesion [TEN, Steven-Johnson syndrome (SJS), erythema multiforme] (Table 1). Including our present case, five of the seven were female. Culprit drugs were ibuprofen (3 cases), acetaminophen (2 cases), antibiotics (trimethoprim-sulfamethazole 1 case, cefdinir 1 case), and carbamazepine (1 case). VBDS with SJS has been reported in 3 children, in 2 children with TEN, and in 2 children with erythema multiforme. The interval between drug intake and VBDS development was variable: one patient had a history of drug-associate erythema multiforme 12 months before the episode of VBDS.11 The prognosis among those cases was also variable. In five of the seven cases, immunosuppressive drug was administrated. Despite immunosuppressive therapy, two cases showed an irreversible change of the intrahepatic bile duct,1,4 three cases improved after immunosuppressive treatment,2,7,12 and two cases, including our case, showed a favorable clinical course without immunosuppressive therapy.11 Further studies are needed to determine the risk group, effective treatment and overall mortality in children with drug-induced VBDS with skin involvement.
In conclusion, a rare case of ibuprofen-associated VBDS with TEN in an infant is presented. Ibuprofen is considered to be among the safest NSAID and generally well tolerated. Nevertheless, clinicians need to be aware of serious acute liver and cutaneous injury as rare adverse effects of this drug.
Figures and Tables
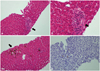 | Fig. 1Liver histology shows portal lymphocytic infiltration with destruction of interlobular bile ducts (H&E, ×200) (A), (H&E, ×400) (B), intralobular canalicular cholestasis (H&E, ×200) (C), and absence of bile ducts demonstrated by cytokeratin 7 immunohistochemical staining (IHC, ×200) (D). |
References
1. Srivastava M, Perez-Atayde A, Jonas MM. Drug-associated acute onset vanishing bile duct and Stevens-Johnson syndromes in a child. Gastroenterology. 1998; 115:743–746.

2. Taghian M, Tran TA, Bresson-Hadni S, Menget A, Felix S, Jacquemin E. Acute vanishing bile duct syndrome after ibuprofen therapy in a child. J Pediatr. 2004; 145:273–276.

3. Degott C, Feldmann G, Larrey D, Durand-Schneider AM, Grange D, Machayekhi JP, et al. Drug-induced prolonged cholestasis in adults: a histological semiquantitative study demonstrating progressive ductopenia. Hepatology. 1992; 15:244–251.

4. Karnsakul W, Arkachaisri T, Atisook K, Wisuthsarewong W, Sattawatthamrong Y, Aanpreung P. Vanishing bile duct syndrome in a child with toxic epidermal necrolysis: an interplay of unbalanced immune regulatory mechanisms. Ann Hepatol. 2006; 5:116–119.

5. Okan G, Yaylaci S, Peker O, Kaymakoglu S, Saruc M. Vanishing bile duct and Stevens-Johnson syndrome associated with ciprofloxacin treated with tacrolimus. World J Gastroenterol. 2008; 14:4697–4700.

6. Morelli MS, O'Brien FX. Stevens-Johnson Syndrome and cholestatic hepatitis. Dig Dis Sci. 2001; 46:2385–2388.
7. Garcia M, Mhanna MJ, Chung-Park MJ, Davis PH, Srivastava MD. Efficacy of early immunosuppressive therapy in a child with carbamazepine-associated vanishing bile duct and Stevens-Johnson syndromes. Dig Dis Sci. 2002; 47:177–182.
8. Stine JG, Lewis JH. Drug-induced liver injury: a summary of recent advances. Expert Opin Drug Metab Toxicol. 2011; 7:875–890.

9. Sternlieb P, Robinson RM. Stevens-Johnson syndrome plus toxic hepatitis due to ibuprofen. N Y State J Med. 1978; 78:1239–1243.
10. Alam I, Ferrell LD, Bass NM. Vanishing bile duct syndrome temporally associated with ibuprofen use. Am J Gastroenterol. 1996; 91:1626–1630.
11. Takeyama J, Saito T, Itagaki T, Abukawa D. Vanishing bile duct syndrome with a history of erythema multiforme. Pediatr Int. 2006; 48:651–653.

12. Tajiri H, Etani Y, Mushiake S, Ozono K, Nakayama M. A favorable response to steroid therapy in a child with drug-associated acute vanishing bile duct syndrome and skin disorder. J Paediatr Child Health. 2008; 44:234–236.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download