Abstract
Purpose
Target-controlled infusion (TCI) of remifentanil can suppress coughing during emergence from general anesthesia; nevertheless, previous studies under different clinical conditions recommend significantly different effective effect-site concentrations (effective Ce) of remifentanil for 50% of patients (EC50). The differences among these studies include type of surgery and patient sex. In recent years, study of sex differences in regards to anesthetic pharmacology has drawn greater interest. Accordingly, we attempted to determine the effective Ce of remifentanil for preventing cough for each sex under the same clinical conditions.
Materials and Methods
Twenty female and 25 male ASA physical status I-II grade patients between the ages of 20 and 46 years who were undergoing thyroidectomy were enrolled in this study. The effective Ce of remifentanil for preventing cough was determined for each sex using the isotonic regression method with a bootstrapping approach, following Dixon's up-and-down method.
Results
Isotonic regression with a bootstrapping approach revealed that the estimated EC50 of remifentanil for preventing coughing during emergence was significantly lower in females {1.30 ng/mL [83% confidence interval (CI), 1.20-1.47 ng/mL]} than in males [2.57 ng/mL (83% CI, 2.45-2.70 ng/mL)]. Mean EC50 in females was also significantly lower than in males (1.23±0.21 ng/mL vs. 2.43±0.21 ng/mL, p<0.001). Mean arterial pressure, heart rate, and respiratory rate over time were not significantly different between the sexes.
Airway reflexes, including coughing, are commonly considered normal responses during emergence from general anesthesia.1,2,3 However, coughing during emergence may lead to a number of potentially dangerous side effects, including laryngospasm, detrimental hemodynamic changes, and increased intraocular and intracranial pressure. Reportedly, post-thyroidectomy bleeding resulting in acute airway obstruction or re-operation occurs in 1-4% of patients,4,5,6,7 and severe cough may cause such bleeding.4,7 In addition to surgeon-related factors, anesthetic factors may also assist in prevention of post-thyroidectomy bleeding. A smooth extubation without significant coughing or retching to avoid raised venous or arterial pressures are important considerations in minimizing the risks of postoperative hemorrhage.7 During awakening from endotracheal anesthesia, the trachea may be stimulated by endotracheal tubes, by noxious effects of the anesthetic gas itself, or by uncleared secretions.3 The presence of an endotracheal tube during emergence from general anesthesia is often associated with severe cough.3,8 Therefore, prevention of cough during emergence from general anesthesia with the presence of a tracheal tube may be important in patients undergoing certain operations.
Intravenous opioids delivered at the end of surgery can facilitate smooth emergence by reducing coughing9,10 and attenuate deleterious hemodynamic changes.11 Specifically, administration of remifentanil via a target-controlled infusion (TCI) makes it possible to reach a defined target concentration without concern that remifentanil levels will rapidly change or increase beyond the intended range.12 Several previous studies, differing in type of surgery, combined anesthetics, and patient sex, have demonstrated reductions of airway reflexes during emergence with the use of remifentanil TCI;13,14 however, the recommended effective effect-site concentrations (effective Ce) of remifentanil for 50% of patients (EC50) reported in these studies range from 1.46 ng/mL to 2.35 ng/mL.13,14
Numerous animal and human studies suggest that sex may affect opioid analgesia: that is, males require higher doses of opioids to achieve similar levels of analgesia as females.15,16,17 This sex difference is known to occur mainly with mu- and kappa-receptor agonists. Sex differences in appropriate opioid dose may affect not only the analgesic system but also act on other inherent properties of the endogenous opioid receptor system, such as respiratory function.18 Mu- and kappa-opioid receptors in the brainstem contribute to regulation of the cough reflex.19 If there is the significant sex difference in remifentanil concentration for airway suppression during anesthetic emergence, a dose regimen suitable for one sex could be excessive for the other, and such information would be clinically important not only in respect of analgesic effect but also in preventing complications.
Accordingly, we hypothesized that men may require higher concentrations of remifentanil for cough suppression during anesthetic emergence than women. The purpose of this study was to determine the EC50 of remifentanil in effect-site TCI for preventing cough in males and females under the same clinical conditions and evaluate whether there are sex differences in EC50 of remifentanil for emergence cough suppression.
This study was approved by the Institutional Review Board of Severance Hospital, Yonsei University Health System (Ref: 4-2011-0356) and registered at http://ClinicalTrials.gov (Ref: NCT01614535). Written informed consent was obtained from all subjects. We enrolled 30 male and 23 female patients of ages 20 to 46 years and of ASA I-II status, all of whom underwent general anesthesia for elective thyroidectomy. Exclusion criteria included patients who exhibited signs of a difficult airway, upper respiratory infection in the previous two weeks, hypertension, or diabetes mellitus. Current smokers were excluded, and female patients who were pregnant, breast-feeding, or menopausal were also excluded. In addition, we withdrew patients who experienced delayed emergence and had not regained consciousness by 15 min after the main anesthetic agent had been stopped.
The patients were not premedicated. After arrival at the operating room, routine monitoring, including electrocardiogram, peripheral oxygen saturation, non-invasive arterial pressure, end-tidal carbon dioxide (EtCO2), and nasopharyngeal temperature, were performed at 1-5 min intervals. Bispectral index (BIS) (BIS® monitor, Covidien Medical, Boulder, CO, USA) monitoring was also performed. Anesthesia was induced using propofol 1.5-2.5 mg/kg I.V. and remifentanil TCI. For TCI of remifentanil, a commercial TCI pump (Orchestra® Base Primea, Fresenius Vial, France) was used, and pump operation was based on the pharmacokinetic model of Minto, et al.20 After non-response to verbal orders and loss of eyelid reflex, rocuronium 0.6 mg/kg I.V. was administered. Tracheal intubation was performed in all patients using a 6.5 mm (internal diameter) tracheal tube for females and a 7.5 mm tracheal tube for males. Cuff pressure was maintained at 20-25 cmH2O using a hand pressure gauge (Hi-Lo™ Hand Pressure Gauge, VBM Medizintechnik GmbH, Germany). Anesthesia was maintained with sevoflurane at 1.5-2.5 vol% in an air/oxygen mixture (FIO2: 0.5, 2 L/min) to achieve a BIS value of 40-55, and a remifentanil Ce of 2-5 ng/mL was used to maintain patients' mean arterial pressure (MAP) and heart rate (HR) within 20% of baseline values. Mechanical ventilation was maintained with a tidal volume of 8 mL/kg, and ventilator frequency was adjusted to maintain EtCO2 at 35-40 mm Hg. Nasopharyngeal temperature was maintained at 36-37℃.
Three anesthetists carried out this study. The first conducted anesthetic induction, maintenance, and control of remifentanil concentration during the emergence period. The second, who did not know the remifentanil concentration during emergence, led anesthetic emergence and extubation. The third anesthetist recorded all variables of interest to this study, including cough grading and was blinded to the Ce of remifentanil. Ten min before the end of surgery, sevoflurane was adjusted to an approximate BIS level of 60, and the remifentanil Ce was titrated to a pre-determined concentration (the initial Ce of remifentanil being 2 ng/mL). This pre-determined Ce was maintained for at least 15 min throughout emergence. When the operation was completed, 30 mg of ketorolac I.V. and 4 mg of ondansetron I.V. were given, and 0.004 mg/kg of glycopyrrolate I.V. and 0.02 mg/kg of neostigmine I.V. were given to reverse neuromuscular block. Simultaneously, sevoflurane was discontinued, and fresh gas flow was increased to 10 L/min. Mechanical ventilation was converted to manual ventilation 3 min after sevoflurane discontinuation, and EtCO2 was maintained at 45-55 mm Hg. During this phase, the patients were not disturbed, except for a verbal request to open their eyes. When the patients opened their eyes, deep breathing was encouraged. The tracheal tube was pulled when spontaneous respiration with an adequate tidal volume was confirmed. Immediately after extubation, remifentanil infusion was stopped, and oxygen was supplemented via a facemask for at least 10 min.
Emergence cough was defined as the cough occurring from the time sevoflurane was turned off to 5 min after extubation. The level of cough was assessed and recorded by the following cough grading system: Grade 0, no cough; Grade 1, single cough with mild severity; Grade 2, cough persistence less than 5 s with moderate severity; or Grade 3, severe, persistent cough for more than 5 s. For estimation of the effective remifentanil Ce, the up-and-down sequential allocation design was used21 (i.e., the predetermined Ce of remifentanil was determined according to the cough response of the previous patient). If the patient did not cough throughout the peri-extubation period, the pre-determined concentration of remifentanil for the subsequent patient was decreased by 0.4 ng/mL. Similarly, if the patient coughed at any time before, during, or after extubation, the pre-determined concentration was increased by 0.4 ng/mL. The upper concentration was limited to 3.0 ng/mL due to concerns for patient safety after extubation.8,14 The stopping rule, which required at least six pairs of failure/success, necessitated the recruitment of more than 20 patients.22 This up-and-down method was conducted independently for each sex.
The MAP and HR were recorded at the following time points: T1, before induction of anesthesia (baseline); T2, end of surgery; T3, before extubation; T4, just after extubation; T5, 5 min after extubation; T6, 10 min after extubation; T7, before transfer out from the postanesthetic care unit. If MAP <60 mm Hg or HR <50 beats per min (beats/min), we injected ephedrine 4 mg I.V. or atropine 0.5 mg I.V. immediately. In addition, respiratory rate (RR) was recorded at the T4-7 points. The time to eye opening (sevoflurane off to eye opening) and time to extubation (sevoflurane off to extubation) were also recorded. Bradypnea, which was defined as a RR <8 breaths per min (bpm), and SpO2 below 95% despite oxygen supplementation were monitored during the entire emergence period.
Patient data are presented as mean±standard deviation, or median and range, or numbers of patients. According to previous studies in which the EC50 was estimated by the Dixon's method,13 the stopping rule required at least six pairs of failure/success. The EC50 of remifentanil was defined as the mean value of the independent crossover pairs for each sex, and we compared the mean EC50s using a t-test. To specify the precision of the target concentration, the isotonic regression method was also used for estimating EC50 and EC95 along with a confidence interval (CI).23 From an observed response rate, which represents the ratio of the number of successful patients to the number of subjects at each concentration level, an adjusted response probability was calculated by pooled adjacent-violators algorithm (PAVA) in order to adhere to the assumption in dose-response determinations that drug effect increases with increased dosage. The CI was estimated by a boot strapping approach.22,24 If the value of EC50 did not overlap at the level of 83% CI, the null hypothesis of equal concentration could be rejected as a type I error of 0.05.25 Other normally distributed variables were analyzed using t-tests. Chi-square tests were used for intergroup comparisons of ordinal variables. Statistical analyses were performed using SAS (version 9.2, SAS Inc., Cary, NC, USA), and all p-values less than 0.05 were considered significant.
Twenty-three female patients and 30 male patients were enrolled in the study. Among the enrolled patients, three female patients were excluded due to surgical factors (nerve injury and delayed skin suture) and an incorrect pre-determined concentration of remifentanil. Five male patients were excluded due to surgical factors (converted from simple thyroidectomy to modified radical neck dissection) and delayed emergence. The patients' characteristics are presented in Table 1. Age was comparable between the females and males; however, due to sex differences, patients' height, weight, body surface area, and body mass index were significantly lower in females. Also, the number of patients for each cough grade was comparable between the females and males.
The up-and-down results in consecutive patients are shown in Fig. 1. The EC50 of remifentanil needed to prevent cough during emergence based on Dixon's method was significantly less in females (1.23±0.21 ng/mL) than in males (2.43±0.21 ng/mL) (p<0.001). The use of isotonic regression estimator, with CIs derived by a bootstrapping approach, revealed that the EC50 of remifentanil was significantly lower in females than in males [1.30 ng/mL (83% CI, 1.20-1.47 ng/mL) vs. 2.57 ng/mL (83% CI, 2.45-2.70 ng/mL)]. Similarly, the EC95 was significantly lower in females than in males [1.86 ng/mL (95% CI, 1.56-1.96 ng/mL) vs. 2.96 ng/mL (95% CI, 2.77-2.98 ng/mL)] (Table 2). PAVA response rates of both sexes are presented in Fig. 2.
RR was 6.2, 9.1, 11.2, and 13.8 bpm at the T4-7 points, respectively, and RR at T5 and T6 points was significantly faster in females, though there was no difference of RR pattern between sexes over time (p-value=0.28) (Fig. 3). In females, bradypnea, defined as a RR <8 bpm, was observed just after extubation (T4 point) in two of seven patients with a Ce of 1.6 ng/mL. In males, bradypnea was observed in two of 10 patients with a Ce of 2.4 ng/mL, in one of nine patients with a Ce of 2.8 ng/mL, and in one of two patients with a Ce of 3.0 ng/mL. One male patient with a Ce of 2.8 ng/mL developed upper airway obstruction and experienced a brief desaturation episode. All of these patients who had adverse respiratory events returned to a normal respiratory pattern within 5 min by encouragement of deep breathing via facial mask without ventilatory support.
The time to eye opening was significantly shorter in females (6.50±1.64 min) than in males (10.01±4.43 min) (p<0.001). The time to extubation was also significantly shorter in females (7.66±1.91 min) than in males (11.27±4.95 min) (p<0.001) (Table 3).
MAP and HR over time did not differ significantly between the sexes [p-value=0.35 (MAP) and 0.082 (HR)] (Fig. 3). The MAP of females was significantly lower than that of males at the T2, T6 and T7 points. HR did not differ between sexes at any time point.
In the present study, we found that the remifentanil requirement for cough suppression during anesthetic emergence in 50% patients was 1.30 ng/mL in females, which was about half of the concentration in males (2.57 ng/mL).
Several studies have investigated the effective Ce of remifentanil for preventing airway reflexes during anesthetic emergence. Jun, et al.26 demonstrated that 1.5 ng/mL of remifentanil effectively suppressed cough during emergence from sevoflurane-remifentanil anesthesia for thyroid surgery. However, Chen, et al.8 reported that 2-2.5 ng/mL was needed to achieve the antitussive effect of remifentanil, following propofol anesthesia for nasal surgery. The most notable difference between the two studies was the type of surgery; in addition, the proportion of male patients in the former study was 14%, whereas it was 62% in the latter. It is not surprising that the opioid requirements differed by sex, but other confounding factors should be excluded. Therefore, we conducted the present study for sex comparison only. Based on our results, the concentrations required for cough suppression during anesthetic emergence under the same clinical conditions were significantly different between sexes. One interesting point is that the cough sensitivity of females was lower than that of males. Dicpinigaitis and Rauf27 reported that healthy women have a more sensitive cough reflex than healthy men. Nevertheless, the antitussive effect was achieved at a lower concentration of remifentanil in females than in males. Male patients with a Ce of 2.0 ng/mL will definitely suffer from severe cough during emergence, while female patients with the same Ce will never cough.
In recent years, there has been growing interest in studying sex differences in anesthetic pharmacology. Compared to males, females are less sensitive to the anesthetic effects of propofol28,29 and ropivacaine,30 but are more sensitive to vecuronium31,32 and rocuronium.33 Of the anesthetic drugs, morphine is the most commonly studied with regard to sex differences, and several studies have shown that females are more sensitive to the analgesic effects of morphine than are males.34,35 Sex differences related to opioids are not restricted to analgesic properties, but are also present in other opioid-mediated responses, including the respiratory system. For instance, morphine has a greater effect on decreasing ventilatory response to carbon dioxide and hypoxic sensitivity in females than in males.18 Most of these studies did not measure morphine or morphine glucuronide concentration and pharmokinetic differences could not be excluded; nevertheless, the major difference is thought to be pharmacodynamic.17
Remifentanil TCI was derived from the results of pharmacokinetic and pharmacodynamic studies, which demonstrated that there are no sex differences related to pharmacokinetic or electroencephalogram (EEG) variables.20 However, there is a lack of studies about the differential effect of remifentanil based on sex,36 and the clinical effects of opioids may differ from EEG variables. Experimental studies for mu-opioid receptor binding, measured by positron emission tomography (PET), illustrated that premenopausal females have significantly higher mu-receptor binding potential than males in the cortical and sub-cortical areas.37 Similarly, Zubieta, et al.38 found that tonic experimental pain produced a greater decrease in mu-opioid receptor availability in several brain regions among men compared to women, apparently due to increased pain-induced binding of endogenous ligand to the receptor. Therefore, the authors suggested that the higher mu-opioid receptor availability among females might explain their increased analgesic responses to exogenous opioids. This pharmacodynamic difference can be expected in remifentanil also, and our results did demonstrate a sex difference for cough suppression.
Several clinical studies have reported respiratory complications at concentrations over 2.5 ng/mL (Ce of remifentanil),8,14 and our research did as well. Though the respiratory complications were not severe and resolved spontaneously or with minimal treatment, further studies regarding the safety of the suggested EC95 are necessary for generalized application of high concentrations of remifentanil.
There are some limitations to the present study. First, we did not measure plasma concentrations of remifentanil. Rather, we calculated the predicted value using Minto's pharmacokinetic model, which is widely used in clinical settings with acceptable levels of bias and inaccuracy.39 Second, the types of surgery included were limited. Thyroid surgery may involve more tracheal irritation than other surgeries, and therefore, caution is needed in extrapolating the findings of this study to other types of surgery.
In conclusion, when remifentanil TCI is used for cough prevention during anesthetic emergence, patient sex may be an important factor for the determination of optimal remifentanil concentrations. Overall, females require lower concentrations of remifentanil for this purpose than do males.
Figures and Tables
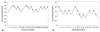 | Fig. 1Assessment of success or failure of smooth emergence over the predetermined concentration of remifentanil based on consecutive patients by Dixon's up-and-down method. Mean EC50 for smooth emergence was calculated from cross-over pairs from failure (open circle) to success (closed circle) in 25 male patients (A) and 20 female patients (B). EC50, effective effect-site concentration (Ce) of remifentanil for suppression of emergence cough in 50% of patients. |
 | Fig. 2Pooled-adjacent-violators algorithm response rate in female (▲) and male (●) groups. EC50 of remifentanil in females was 1.30 ng/mL (83% CI, 1.20-1.47 ng/mL) and 2.57 ng/mL (83% CI, 2.45-2.70 ng/mL) in males. EC95 in females was 1.86 ng/mL (95% CI, 1.56-1.96 ng/mL) and 2.96 ng/mL (95% CI, 2.77-2.98 ng/mL) in males. Both EC50 and EC95 were significantly lower in the female group than in the male group. EC50, effective Ce of remifentanil for suppression of emergence cough in 50% of patients; EC95, effective Ce of remifentanil for suppression of emergence cough in 95% of patients; CI, confidence interval. |
 | Fig. 3MAP, HR, and RR were not significantly different in females (▲) and males (●) over time (p-values for MAP, HR, and RR=0.351, 0.082 and 0.277, respectively). The female MAP was significantly higher than that of males at the end of the surgery, 10 min after extubation, and before transfer out from the postanesthetic care unit (p=0.012, p<0.001, and p<0.001, respectively). With regard to HR, there was no significant difference at any time point. RR increased continuously from each prior time point in both males and females: just after extubation to 5 min after extubation (p<0.001), 5 min after extubation to10 min after extubation (p<0.001), and 10 min after extubation to before transfer out from the post-anesthetic care unit (p<0.001). MAP, mean arterial pressure; HR, heart rate; RR, respiratory rate; bpm, breaths per min. |
Table 2
EC50 and EC95 of Remifentanil for Emergence without Cough

PAVA, pooled-adjacent-violators algorithm; CI, confidence interval; EC50, effective Ce of remifentanil for suppression of emergence cough in 50% of patients; EC95, effective Ce of remifentanil for suppression of emergence cough in 95% of patients.
Values are presented as the mean (83%* or 95%† CI). EC50 and EC95 were calculated by isotonic regression method using the PAVA, and CIs were calculated using a bootstrapping approach.
ACKNOWLEDGEMENTS
The authors thank Hye Sun Lee, Biostatistics Collaboration Unit, Yonsei University College of Medicine.
Notes
References
1. Miller KA, Harkin CP, Bailey PL. Postoperative tracheal extubation. Anesth Analg. 1995; 80:149–172.
2. Soltani HA, Aghadavoudi O. The effect of different lidocaine application methods on postoperative cough and sore throat. J Clin Anesth. 2002; 14:15–18.

3. Kim ES, Bishop MJ. Cough during emergence from isoflurane anesthesia. Anesth Analg. 1998; 87:1170–1174.
4. Lee HS, Lee BJ, Kim SW, Cha YW, Choi YS, Park YH, et al. Patterns of Post-thyroidectomy Hemorrhage. Clin Exp Otorhinolaryngol. 2009; 2:72–77.

5. Godballe C, Madsen AR, Pedersen HB, Sørensen CH, Pedersen U, Frisch T, et al. Post-thyroidectomy hemorrhage: a national study of patients treated at the Danish departments of ENT Head and Neck Surgery. Eur Arch Otorhinolaryngol. 2009; 266:1945–1952.

6. Leyre P, Desurmont T, Lacoste L, Odasso C, Bouche G, Beaulieu A, et al. Does the risk of compressive hematoma after thyroidectomy authorize 1-day surgery? Langenbecks Arch Surg. 2008; 393:733–737.

7. Harding J, Sebag F, Sierra M, Palazzo FF, Henry JF. Thyroid surgery: postoperative hematoma--prevention and treatment. Langenbecks Arch Surg. 2006; 391:169–173.

8. Chen J, Li W, Wang D, Hu X. The effect of remifentanil on cough suppression after endoscopic sinus surgery: a randomized study. Acta Anaesthesiol Scand. 2010; 54:1197–1203.

9. Tagaito Y, Isono S, Nishino T. Upper airway reflexes during a combination of propofol and fentanyl anesthesia. Anesthesiology. 1998; 88:1459–1466.

10. Kim JM, Lee JH, Lee HJ, Koo BN. Comparison of emergence time in children undergoing minor surgery according to anesthetic: desflurane and sevoflurane. Yonsei Med J. 2013; 54:732–738.

11. Nishina K, Mikawa K, Maekawa N, Obara H. Fentanyl attenuates cardiovascular responses to tracheal extubation. Acta Anaesthesiol Scand. 1995; 39:85–89.

12. Egan TD. Target-controlled drug delivery: progress toward an intravenous "vaporizer" and automated anesthetic administration. Anesthesiology. 2003; 99:1214–1219.
13. Lee B, Lee JR, Na S. Targeting smooth emergence: the effect site concentration of remifentanil for preventing cough during emergence during propofol-remifentanil anaesthesia for thyroid surgery. Br J Anaesth. 2009; 102:775–778.

14. Choi EM, Park WK, Choi SH, Soh S, Lee JR. Smooth emergence in men undergoing nasal surgery: the effect site concentration of remifentanil for preventing cough after sevoflurane-balanced anaesthesia. Acta Anaesthesiol Scand. 2012; 56:498–503.

15. Fillingim RB, Gear RW. Sex differences in opioid analgesia: clinical and experimental findings. Eur J Pain. 2004; 8:413–425.

16. Dahan A, Kest B, Waxman AR, Sarton E. Sex-specific responses to opiates: animal and human studies. Anesth Analg. 2008; 107:83–95.

17. Sarton E, Olofsen E, Romberg R, den Hartigh J, Kest B, Nieuwenhuijs D, et al. Sex differences in morphine analgesia: an experimental study in healthy volunteers. Anesthesiology. 2000; 93:1245–1254.
18. Dahan A, Sarton E, Teppema L, Olievier C. Sex-related differences in the influence of morphine on ventilatory control in humans. Anesthesiology. 1998; 88:903–913.

19. Kamei J. Role of opioidergic and serotonergic mechanisms in cough and antitussives. Pulm Pharmacol. 1996; 9:349–356.

20. Minto CF, Schnider TW, Egan TD, Youngs E, Lemmens HJ, Gambus PL, et al. Influence of age and gender on the pharmacokinetics and pharmacodynamics of remifentanil I Model development. Anesthesiology. 1997; 86:10–23.

22. Pace NL, Stylianou MP. Advances in and limitations of up-and-down methodology: a précis of clinical use, study design, and dose estimation in anesthesia research. Anesthesiology. 2007; 107:144–152.
23. Stylianou M, Flournoy N. Dose finding using the biased coin up-and-down design and isotonic regression. Biometrics. 2002; 58:171–177.

24. Dilleen M, Heimann G, Hirsch I. Non-parametric estimators of a monotonic dose-response curve and bootstrap confidence intervals. Stat Med. 2003; 22:869–882.

25. Payton ME, Greenstone MH, Schenker N. Overlapping confidence intervals or standard error intervals: what do they mean in terms of statistical significance? J Insect Sci. 2003; 3:34.

26. Jun NH, Lee JW, Song JW, Koh JC, Park WS, Shim YH. Optimal effect-site concentration of remifentanil for preventing cough during emergence from sevoflurane-remifentanil anaesthesia. Anaesthesia. 2010; 65:930–935.

27. Dicpinigaitis PV, Rauf K. The influence of gender on cough reflex sensitivity. Chest. 1998; 113:1319–1321.

28. Andrade J, Sapsford DJ, Jeevaratnum D, Pickworth AJ, Jones JG. The coherent frequency in the electroencephalogram as an objective measure of cognitive function during propofol sedation. Anesth Analg. 1996; 83:1279–1284.

29. Glass PS, Bloom M, Kearse L, Rosow C, Sebel P, Manberg P. Bispectral analysis measures sedation and memory effects of propofol, midazolam, isoflurane, and alfentanil in healthy volunteers. Anesthesiology. 1997; 86:836–847.

30. Li Y, Zhou Y, Chen H, Feng Z. The effect of sex on the minimum local analgesic concentration of ropivacaine for caudal anesthesia in anorectal surgery. Anesth Analg. 2010; 110:1490–1493.

31. Semple P, Hope DA, Clyburn P, Rodbert A. Relative potency of vecuronium in male and female patients in Britain and Australia. Br J Anaesth. 1994; 72:190–194.

32. Xue FS, Liao X, Liu JH, Tong SY, Zhang YM, Zhang RJ, et al. Dose-response curve and time-course of effect of vecuronium in male and female patients. Br J Anaesth. 1998; 80:720–724.

33. Xue FS, Tong SY, Liao X, Liu JH, An G, Luo LK. Dose-response and time course of effect of rocuronium in male and female anesthetized patients. Anesth Analg. 1997; 85:667–671.

34. Burns JW, Hodsman NB, McLintock TT, Gillies GW, Kenny GN, McArdle CS. The influence of patient characteristics on the requirements for postoperative analgesia. A reassessment using patient-controlled analgesia. Anaesthesia. 1989; 44:2–6.

35. Chia YY, Chow LH, Hung CC, Liu K, Ger LP, Wang PN. Gender and pain upon movement are associated with the requirements for postoperative patient-controlled iv analgesia: a prospective survey of 2,298 Chinese patients. Can J Anaesth. 2002; 49:249–255.

36. Pleym H, Spigset O, Kharasch ED, Dale O. Gender differences in drug effects: implications for anesthesiologists. Acta Anaesthesiol Scand. 2003; 47:241–259.

37. Zubieta JK, Dannals RF, Frost JJ. Gender and age influences on human brain mu-opioid receptor binding measured by PET. Am J Psychiatry. 1999; 156:842–848.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download