Abstract
Purpose
This analysis was done to investigate the optimal regimen for fentanyl-based intravenous patient-controlled analgesia (IV-PCA) by finding a safe and effective background infusion rate and assessing the effect of adding adjuvant drugs to the PCA regimen.
Materials and Methods
Background infusion rate of fentanyl, type of adjuvant analgesic and/or antiemetic that was added to the IV-PCA, and patients that required rescue analgesics and/or antiemetics were retrospectively reviewed in 1827 patients who underwent laparoscopic abdominal surgery at a single tertiary hospital.
Results
Upon multivariate analysis, lower background infusion rates, younger age, and IV-PCA without adjuvant analgesics were identified as independent risk factors of rescue analgesic administration. Higher background infusion rates, female gender, and IV-PCA without additional 5HT3 receptor blockers were identified as risk factors of rescue antiemetics administration. A background infusion rate of 0.38 µg/kg/hr [area under the curve (AUC) 0.638] or lower required rescue analgesics in general, whereas, addition of adjuvant analgesics decreased the rate to 0.37 µg/kg/hr (AUC 0.712) or lower. A background infusion rate of 0.36 µg/kg/hr (AUC 0.638) or higher was found to require rescue antiemetics in general, whereas, mixing antiemetics with IV-PCA increased the rate to 0.37 µg/kg/hr (AUC 0.651) or higher.
Conclusion
Background infusion rates of fentanyl between 0.12 and 0.67 µg/kg/hr may safely be used without any serious side effects for IV-PCA. In order to approach the most reasonable background infusion rate for effective analgesia without increasing postoperative nausea and vomiting, adding an adjuvant analgesic and an antiemetic should always be considered.
Of various methods that are used for acute postoperative pain control, intravenous patient-controlled analgesia (IV-PCA) is the most commonly employed modality in modern medicine.1 Despite the lack of consensus on the appropriate dose of opioids or the use of adjuvants, IV-PCA has already become standard practice for postoperative pain control in most hospitals. Moreover, it has been reported that most patients prefer IV-PCA over other methods, with high satisfaction scores.2,3
Many studies have been undertaken to determine the ideal IV-PCA regimen that would maximize pain relief and minimize opioid-related side effects at the same time.1,4,5 Whether or not to use a background infusion rate or add an adjuvant drug to the IV-PCA are among the most debated issues regarding the application of IV-PCA. However, most clinical studies dealing with these issues were done with morphine-based regimens, as it is the most commonly used opioid.1,4 Although fentanyl is considered as appropriate and perhaps better suited than morphine for IV-PCA due to its rapid onset and short duration of action,4,6 there is a relative shortage of evidence regarding its proper use in IV-PCA.
The department of anesthesia of our hospital has been using fentanyl-based IV-PCA with background infusion for several years. However, PCA regimens were liberally decided by the attending anesthesiologist's preference and judgment, which resulted in a wide range of background infusion rates of fentanyl and different mixtures of analgesics and antiemetics. Due to this heterogeneity of IV-PCA regimens, a question of whether there was a significant difference in analgesic efficacy or complication rates between regimens with different background infusion rates or adjuvant drugs remains largely unclear. This retrospective analysis attempted to analyze the regimens that were used for IV-PCA in a large number of patients who underwent laparoscopic abdominal surgery at a single tertiary hospital, and investigate a more optimal regimen for fentanyl-based IV-PCA in terms of background infusion rates and adjuvant drugs.
This study is a retrospective analysis of patients who received a fentanyl-based IV-PCA with background infusion after laparoscopic surgery. The study protocol was approved by the Institutional Review Board and Hospital Research Ethics Committee of Severance Hospital. The data used for this study was from a database which was prospectively compiled by a dedicated PCA management team that consisted of two trained nurses. The PCA management team was trained to do ward rounds twice a day in every patient who was connected to any form of PCA device after surgery in order to evaluate the quality of pain control, need for additional rescue analgesics or antiemetics and the presence of any side effects. A sedation level of 3 or 4 on the Pasero Opioid-Induced Sedation Scale7 was defined as unacceptable sedation, and respiratory depression was defined as ventilator frequency of less than 10 breaths per minute or oxygen saturation less than 90% when monitoring was applied. Rescue drugs were given according to the hospital protocol. Patients were administered rescue analgesics when reported pain scores were higher than 4 on a 10 mm visual analogue scale (0: no pain, 10: worst imaginable pain) or upon patient request. Ketorolac (Keromin®, Hana Pharm. Co., Seoul, Korea) 30 mg, or pethidine (Pethidine®, Jeil Pharmaceutical Co. Ltd., Daegu, Korea) 25 mg were given as rescue analgesics depending on pain character and patient characteristics. Rescue antiemetics were given when patients reported nausea scores higher than 4 on an 11-point verbal numerical rating scale (0: no nausea, 10: worst imaginable nausea), when retching or vomiting developed or by patient request. Metoclopramide (Macperan®, Dong Wha Pharm. Co., Ltd., Seoul, Korea) 10 mg was given as the 1st line rescue antiemetics, and patients with persistent and refractory postoperative nausea and vomiting (PONV) were given 5-HT3 receptor antagonists such as ondansetron (Onseran®, Yuhan, Seoul, Korea) 4 mg or ramosetron (Nasea®, Astellas Pharma Korea, Seoul, Korea) 0.3 mg.
A total of 2548 consecutive patients who used a fentanyl-based IV-PCA after laparoscopic abdominal surgery between September 2010 and August 2012 were identified and screened for eligibility for analysis. Among these patients, only those who had an IV-PCA device programmed to use 2 mL/hr for background infusion, a demand volume of 0.5 mL, and a lock-out interval of 15 minutes with a total volume of 100 mL were included in this study. Exclusion criteria included patients who required postoperative intensive care unit (ICU) care, and patients who were administered routine analgesics and antiemetics on a regular basis. Patient age, gender, body mass index (BMI), American Society of Anesthesiologist class, comorbidities, additional risk factors of PONV (e.g., history of smoking, motion sickness and PONV) and anesthesia duration were analyzed by electronic medical record review. The background infusion rates of fentanyl, type of adjuvant analgesic and/or antiemetic that were added to the IV-PCA regimen and patients who required rescue analgesics and/or antiemetics were also reviewed and analyzed according to the PCA management team database. We also identified independent risk factors of rescue analgesic or antiemetic administration during postoperative 48 hours. Based on the PCA-related data that was collected, we analyzed and compared the cutoff value of fentanyl background infusion rate that would require rescue analgesics or antiemetics between patients who received IV-PCA with or without adjuvant analgesics or antiemetics.
Continuous variables are shown as mean±SD and categorical variables are shown as numbers (percentage). To identify independent predictors of rescue analgesic or antiemetic administration, a logistic regression model was used. Potential confounding factors for analysis were selected on the basis of literature review and included demographic data (gender, age, BMI), underlying medical diseases (hypertension, diabetes mellitus), known risk factors of PONV (history of smoking, motion sickness and PONV) and anesthesia duration. Background infusion rate and the factors of whether adjuvant analgesics or antiemetics were added in the IV-PCA were also added in the analysis. First, univariate logistic regression analysis was performed to identify significant predictors of rescue analgesic or antiemetic administration using the aforementioned variables. The factors that had a p-value of <0.05 were then included in the multivariate logistic regression analysis, as well as the demographic data, known risk factors of PONV, anesthesia duration, background infusion rate and the presence of adjuvant analgesics or antiemetics in the IV-PCA. Odds ratios and associated 95% confidence intervals were estimated. Receiver operating characteristic (ROC) curve analysis was performed to obtain cutoff values of background infusion rates that would require rescue analgesics and antiemetics. Optimal cutoff values were determined on the basis of the maximum values of the Youden index, calculated by [sensitivity+specificity-1]. All analyses were performed with SPSS for Windows version 20.0 (IBM SPSS Inc., Chicago, IL, USA). p<0.05 was considered to be significant.
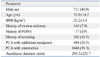
Of the 2548 consecutive patients that were identified, 434 patients who used a background infusion rate of 1 mL/hr, 118 patients who required ICU care, and an additional 169 patients who were administered routine analgesics and antiemetics on a regular basis were excluded, leaving 1827 patients eligible for analysis. The flowchart for patient sample selection is shown in Fig. 1. Patient characteristics which include demographic data, risk factors for PONV and PCA-related data are shown in Table 1. Of the 1827 patients, 484 (26.5%) patients received an IV-PCA containing adjuvant analgesics. 455 (94.0%) of these patients received 90 to 120 mg of ketorolac (Keromin®, Hana Pharm. Co., Seoul, Korea) and the remaining 29 (6.0%) received 120 to 160 mg of nefopam (Acupan®, Pharmbio Korea, Seoul, Korea). 5HT3 receptor antagonists were added as antiemetics in 1668 (91.3%) patients; 1037 (62.2%) of these patients received 8 to 12 mg of ondansetron (Onseran®, Yuhan, Seoul, Korea) and 631 (37.8%) received 0.3 to 0.6 mg of ramosetron (Nasea®, Astellas Pharma Korea, Seoul, Korea). The background infusion rate of fentanyl ranged between 0.12 and 0.67 µg/kg/hr.
The number of patients who required rescue analgesics at least once during the post-operative 48 hour period were 1464 (80.1%), and 1063 (58.2%) patients were administered multiple doses of analgesics. The number of patients who were administered rescue analgesics was highest during the postoperative 1 to 6 hour period and gradually decreased thereafter. The mean frequency of rescue analgesic administration per patient was highest during the postoperative 1 to 6 hour period. PONV was reported in 467 (25.6%) patients, and 275 (15.1%) patients required rescue antiemetics. 88 (4.8%) patients were administered multiple doses of antiemetics. The number of patients who were administered rescue antiemetics was highest during postoperative 1 to 6 hours. Other side effects such as dizziness, sedation, headache and pruritus were reported in 105 (5.8%), 43 (2.4%), 26 (1.4%) and 16 (0.9%) patients, respectively. Respiratory depression was not found in any of the patients.
Upon multivariate analysis, lower background infusion rates, younger age and IV-PCA without adjuvant analgesics were identified as independent risk factors of rescue analgesic administration (Table 2). Higher background infusion rates, female gender, and IV-PCA without additional 5HT3 receptor blockers were identified as risk factors of rescue antiemetics administration (Table 3).
According to the ROC curve analysis, a background infusion rate of 0.38 µg/kg/hr [area under the curve (AUC) 0.638] or lower required rescue analgesics in general. However, background infusion rates that would require rescue analgesics with and without adjuvant analgesics in the IV-PCA were 0.37 µg/kg/hr (AUC 0.712) or lower and 0.38 µg/kg/hr (AUC 0.619) orlower, respectively (Table 4). A background infusion rate of 0.36 µg/kg/hr (AUC 0.638) or higher was found to require rescue antiemetics in general, while mixing antiemetics to the IV-PCA increased the rate to 0.37 µg/kg/hr (AUC 0.651) or higher. However, a background infusion rate of 0.34 µg/kg/hr (AUC 0.611) or higher was found to require rescue antiemetics without antiemetics in the IV-PCA (Table 5).
Although many clinicians and patients now prefer to use IV-PCA over other modalities for postoperative pain control, there is still a considerable amount of debate over whether or not to use background infusions and combine adjuvant analgesics. Using background infusion for IV-PCA has been discouraged by many due to the risk of respiratory depression without any improvement in pain relief,8 although some found otherwise.9,10 The employment of multimodal analgesia with the intention of achieving opioid-sparing effects has been extensively studied, with inconsistent results.11,12 Our present retrospective analysis showed that a wide range of background infusion rate of fentanyl between 0.12 and 0.67 µg/kg/hr was being used for IV-PCA without causing severe adverse effects. Most importantly, adding both an adjuvant analgesic and an antiemetic to the IV-PCA regimen was found to simultaneously decrease the background infusion rate required for effective pain relief as well as the incidence of PONV.
Most of the previous studies that assessed the safety and efficacy of postoperative IV-PCA with background infusion were conducted in morphine-based regimens.13,14,15, 16 Morphine has been the most widely used opioid in IV-PCA for a long time, and several studies showed evidence of safe and effective background infusion rates of morphine.9,10,17,18 On the other hand, little is known about appropriate background infusion rates of fentanyl when used in postoperative IV-PCA. A highly lipophilic drug without any active metabolites, fentanyl offers a wider therapeutic index than morphine.19 While the use of morphine background infusion is basically criticized and discouraged based on the risk of overmedication and respiratory depression,8,16 the different pharmacokinetic properties of fentanyl may cause the patient to redose frequently or include background infusion as part of the PCA setting.1 In terms of safety, fentanyl has been reported to cause less respiratory depression and other opioid-related side effects compared to morphine.20,21 Two recent studies that employed a background infusion rate of fentanyl in IV-PCA did not find any cases of respiratory depression,22,23 even at a relatively high rate of 0.4 µg/kg/h.23 While these studies provide supportive evidence regarding the use of fentanyl with background infusion in IV-PCA, they were not able to offer any guideline as to how to mix the IV-PCA regimen to achieve both effective pain relief and a low incidence of PONV.
In the present analysis, lower background infusion rate and PCA without adjuvant analgesics were identified as significant risk factors of rescue analgesic administration. However, higher background infusion rates decreased the administration of rescue analgesics, while it reversely increased the risk of rescue antiemetic administration. Naturally, mixing antiemetics in the IV-PCA was found to decrease the risk of rescue antiemetic requirements. Overall, these results offer a rationale to mix both adjuvant analgesics and antiemetics to the IV-PCA in order to improve pain relief and decrease PONV. Combining different classes of analgesics in order to improve pain relief and decrease drug-related adverse effects has been coined 'multimodal analgesia',24 and various drugs have been evaluated as possible adjuvants to opioids in IV-PCA regimens.4,8 Despite inconsistent results and scarcity of large-scale prospective randomized trials, there is a clear trend towards the use of this technique in postoperative pain management25,26 and some authors even recommend that multimodal analgesia should be used whenever possible.27 The risk factor analysis of the present study is in context with this concept, and also shows that adding an antiemetic may decrease the risk of PONV in patients using fentanyl-based IV-PCA.
The results of the present study seem meaningful in that we analyzed the cutoff values of background infusion rates that would not require rescue drugs in patients using fentanyl-based postoperative IV-PCA. While the aforementioned risk factor analysis gave us a broad idea of the importance of adding adjuvant drugs to the fentanyl-based IV-PCA, analyzing the cutoff values was done to find out whether there were actual significant alterations in the postulated optimal background infusion rates when adding or excluding adjuvant drugs. To the best of our knowledge, there are no previous studies that addressed this issue in a large number of patients. We first found that a fentanyl background infusion rate of at least 0.38 µg/kg/h would be required in order to achieve effective pain relief without the need for rescue analgesics in patients using a fentanyl-only IV-PCA. Interestingly, we also found that IV-PCA without antiemetics would cause PONV severe enough to require administration of a rescue antiemetic at background infusion rates of over 0.34 µg/kg/h. Taken together, the worst case scenario would be patients possibly suffering from insufficient pain control and PONV when using a fentanyl-only IV-PCA at background infusion rates between 0.34 and 0.38 µg/kg/h. Moreover, patients using unnecessarily high background infusion rates would be at a risk for discontinuation of the IV-PCA altogether, and uncontrollable PONV could occur. However, the cutoff value of background infusion rate that would require rescue analgesics was reduced from 0.38 µg/kg/h to 0.37 µg/kg/h when an adjuvant drug was added to the IV-PCA. Although this infusion rate still exceeds 0.34 µg/kg/h, the gap between the cutoff values of background infusion that would not require rescue antiemetics and rescue analgesics was narrowed to 0.03 µg/kg/h. Finally, the cutoff value of background infusion that would require rescue antiemetics was increased to 0.37 µg/kg/h when antiemetics were added to the IV-PCA, ultimately eliminating the gap between the two cutoff values. This not only supports the results of the risk factor analysis, but also suggests that a certain background infusion rate of fentanyl may indeed exist for IV-PCA that can maximize pain relief and minimize PONV. While the background infusion rate of 0.37 per se cannot be considered as a definite value that can be directly applied to clinical practice, a well-designed prospective study in the future should be able to offer some insight into a certain infusion rate that may be applied to certain patient populations.
This study has some limitations. First of all, the AUCs of the cut-off values were relatively low due to uneven distribution of background infusion rates of fentanyl and low incidence of PONV. A randomized controlled trial without a skewed pattern of background infusion rates are needed for more supportive evidence. Secondly, the total consumed amount of analgesics per time unit or the frequency and amount of bolus doses on demand could not be analyzed due to retrospective design of the present study. We were only able to evaluate the quality of analgesia by comparing the frequency of rescue analgesic administration as a surrogate. Lastly, the types of adjuvant drugs or surgery were not controlled in the present analysis. A well designed randomized controlled trial should be able to provide more insight into effective procedure-specific regimens in the future.
Important as it is for safe and effective analgesia, it is clear that finding the 'ideal' regimen for fentanyl-based IV-PCA is a difficult task. The results of the present study show that background infusion rates of fentanyl between 0.12 and 0.67 µg/kg/hr may be safely used without any serious side effects such as respiratory depression for IV-PCA. However, in order to approach the most reasonable background infusion rate that can offer effective analgesia without increasing PONV, adding an adjuvant analgesic regardless of its drug class together with an antiemetic should be always considered.
Figures and Tables
Table 1
Characteristics of Patients That Received Postperative Intravenous Patient-Controlled Analgesia
References
1. Palmer PP, Miller RD. Current and developing methods of patient-controlled analgesia. Anesthesiol Clin. 2010; 28:587–599.

2. Ballantyne JC, Carr DB, Chalmers TC, Dear KB, Angelillo IF, Mosteller F. Postoperative patient-controlled analgesia: meta-analyses of initial randomized control trials. J Clin Anesth. 1993; 5:182–193.

3. Walder B, Schafer M, Henzi I, Tramèr MR. Efficacy and safety of patient-controlled opioid analgesia for acute postoperative pain. A quantitative systematic review. Acta Anaesthesiol Scand. 2001; 45:795–804.
4. Momeni M, Crucitti M, De Kock M. Patient-controlled analgesia in the management of postoperative pain. Drugs. 2006; 66:2321–2337.

5. Kim SH, Shin YS, Oh YJ, Lee JR, Chung SC, Choi YS. Risk assessment of postoperative nausea and vomiting in the intravenous patient-controlled analgesia environment: predictive values of the Apfel's simplified risk score for identification of high-risk patients. Yonsei Med J. 2013; 54:1273–1281.

7. Pasero C. Assessment of sedation during opioid administration for pain management. J Perianesth Nurs. 2009; 24:186–190.

9. White I, Ghinea R, Avital S, Chazan S, Dolkart O, Weinbroum AA. Morphine at "sub-analgesic" background infusion rate plus low-dose PCA bolus control pain better and is as safe as twice a bolus-only PCA regimen: a randomized, double blind study. Pharmacol Res. 2012; 66:185–191.

10. Guler T, Unlugenc H, Gundogan Z, Ozalevli M, Balcioglu O, Topcuoglu MS. A background infusion of morphine enhances patient-controlled analgesia after cardiac surgery. Can J Anaesth. 2004; 51:718–722.

11. White PF, Kehlet H. Improving postoperative pain management: what are the unresolved issues? Anesthesiology. 2010; 112:220–225.
13. Fleming BM, Coombs DW. A survey of complications documented in a quality-control analysis of patient-controlled analgesia in the postoperative patient. J Pain Symptom Manage. 1992; 7:463–469.

14. Sidebotham D, Dijkhuizen MR, Schug SA. The safety and utilization of patient-controlled analgesia. J Pain Symptom Manage. 1997; 14:202–209.

15. Schug SA, Torrie JJ. Safety assessment of postoperative pain management by an acute pain service. Pain. 1993; 55:387–391.

16. Hagle ME, Lehr VT, Brubakken K, Shippee A. Respiratory depression in adult patients with intravenous patient-controlled analgesia. Orthop Nurs. 2004; 23:18–27.

17. Pasero C, McCaffery M. Safe use of a continuous infusion with i.v. PCA. J Perianesth Nurs. 2004; 19:42–45.

18. Sam WJ, MacKey SC, Lötsch J, Drover DR. Morphine and its metabolites after patient-controlled analgesia: considerations for respiratory depression. J Clin Anesth. 2011; 23:102–106.

20. Cepeda MS, Farrar JT, Baumgarten M, Boston R, Carr DB, Strom BL. Side effects of opioids during short-term administration: effect of age, gender, and race. Clin Pharmacol Ther. 2003; 74:102–112.

21. Hutchison RW, Chon EH, Tucker JW, Gilder R, Moss J, Daniel P. A Comparison of a Fentanyl, Morphine, and Hydromorphone Patient-Controlled Intravenous Delivery for Acute Postoperative Analgesia: A Multicenter Study of Opioid-Induced Adverse Reactions. Hosp Pharm. 2006; 41:659–663.

22. Kim SY, Kim EM, Nam KH, Chang DJ, Nam SH, Kim KJ. Postoperative intravenous patient-controlled analgesia in thyroid surgery: comparison of fentanyl and ondansetron regimens with and without the nonsteriodal anti-inflammatory drug ketorolac. Thyroid. 2008; 18:1285–1290.

23. Song JW, Park EY, Lee JG, Park YS, Kang BC, Shim YH. The effect of combining dexamethasone with ondansetron for nausea and vomiting associated with fentanyl-based intravenous patient-controlled analgesia. Anaesthesia. 2011; 66:263–267.

25. Fletcher D, Fermanian C, Mardaye A, Aegerter P. Pain and Regional Anesthesia Committee of the French Anesthesia and Intensive Care Society (SFAR). A patient-based national survey on postoperative pain management in France reveals significant achievements and persistent challenges. Pain. 2008; 137:441–451.

26. Kehlet H, Dahl JB. The value of "multimodal" or "balanced analgesia" in postoperative pain treatment. Anesth Analg. 1993; 77:1048–1056.

27. Kehlet H, Werner M, Perkins F. Balanced analgesia: what is it and what are its advantages in postoperative pain? Drugs. 1999; 58:793–797.




 PDF
PDF ePub
ePub Citation
Citation Print
Print







 XML Download
XML Download