Abstract
Purpose
To investigate the therapeutic effectiveness of ultrasound (US)-guided trigger point injection for myofascial trigger points (MTrPs) in the internal rotator muscles of the shoulder in post-mastectomy patients.
Materials and Methods
This pilot study was a non-controlled, prospective, clinical trial. Nineteen post-mastectomy patients with a diagnosis of at least one active MTrP in the subscapularis and/or pectoralis muscles were included. We performed trigger point injections into the subscapularis muscle deep behind the scapula as well as the pectoralis muscle for diagnostic and therapeutic purpose by the newly developed US-guided method.
Results
Visual analogue scale and range of motion of the shoulder for external rotation and of abduction showed significant improvement immediately after the first injection and 3 months after the last injection compared with baseline (p<0.05 for both). Duration from onset to surgery and duration of myofascial pain syndrome in the good responder group were significantly shorter than in the bad responder group (p<0.05). Patients did not report any complications related to the procedure or serious adverse events attributable to the treatment.
Conclusion
In post-mastectomy patients with shoulder pain, US-guided trigger point injections of the subscapularis and/or pectoralis muscles are effective for both diagnosis and treatment when the cause of shoulder pain is suspected to originate from active MTrPs in these muscles, particularly, the subscapularis.
Women diagnosed with breast cancer can expect substantially better survival rates than those with other common cancers, although breast cancer remains the leading form of cancer in women. The high incidence rate along with relatively favorable survival rate makes the quality of survival important.1 Especially, breast cancer patients with shoulder and arm pain have significantly decreased quality of life compared to women without breast cancer, as well as breast cancer patients who do not have shoulder and arm pain.2 Different diagnoses, such as rotator cuff disease, myofascial trigger point (MTrP), adhesive capsulitis, axillary web syndrome, and lateral epicondylitis have been used to describe shoulder and arm pain among breast cancer patients.3,4
An MTrP is a highly localized painful or sensitive spot located in a palpable taut band of skeletal muscle fibers in patients with myofascial pain syndrome (MPS).5 Pain from active MTrPs can occur spontaneously or in response to movement. A latent MTrP is defined as a sensitive spot at which pain or discomfort is elicited by compression only. The diagnosis of MPS, which manifest with one or more active MTrP, usually is based on the patient's subjective symptoms and the presence of an active MTrP characterized by 1) tender spots in one or more palpable taut band, 2) a referred pain pattern, 3) a local twitch response (LTR), and 4) restricted range of motion (ROM).6 Myofascial tissues have been implicated in the origin of shoulder pain.7 In this context, MPS has been a potential cause of chronic pain in breast cancer survivors who have undergone breast cancer surgery.5 In their study, MPS on the pectoralis major, infraspinatus and upper trapezius were highly prevalent.
We have recently observed a significant number of patients with post-mastectomy who were compatible with the criteria of MPS in the subscapularis muscle as well as the pectoralis muscle, as assessed by careful physical examination. However, trigger point injection into the subscapularis is difficult without neurovascular injury or incorrect injection to the muscles other than the subscapularis because of its deep location. An ultrasound (US)-guided injection technique into the suscapularis muscle was introduced for the treatment of spasticity.8 In this approach, the patient assumed the lateral decubitus position with the affected side up and US-guided injections into motor points were performed safely and accurately. However, trigger point injections differ in terms of a multiple number of needlings for variable locations of MTrPs, in contrast to less variable locations of motor points between patients with spastic subscapularis. Therefore, patient positions for motor point injections for spastic subscapularis muscles would not be appropriate for trigger point injections for MTrPs in the subscapularis muscle. Patients with MPS in the shoulder experience less limitation of motion, especially in the scapular upward rotation, compared to spastic shoulders. While the lateral decubitus position is appropriate to expose the subscapularis muscle in the spastic shoulder, it would be sufficient to observe the subscapularis muscle with MTrPs for trigger point injections on ultrasonography, if the patient is placed in the supine position with the shoulder flexed, externally rotated and abducted with the scapula rotated upwardly.9
This study applied a novel position--the supine position with the scapula rotated upwardly--for post-mastectomy patients with MPS in the subscapularis muscles. Hence, this study aimed to investigate the therapeutic effectiveness of US-guided trigger point injection for active MTrPs in the internal rotator muscles of the shoulder in patients who are status post breast cancers surgery. This study was designed to test following hypotheses:
We performed accurate trigger point injections into the subscapularis muscle deep behind the scapula as well as into the pectoralis muscle for the purposes of diagnosis and treatment using the newly developed US-guided method.
The potential participants of this study were women older than 18 years with post-mastectomy who were referred to the physiatrist (S.C.L) for shoulder pain between April 2011 and June 2012. To be eligible for the study, participants fulfilled the following criteria: 1) diagnosis of breast cancer (grades I to IIIB); 2) postmastectomy state for at least 6 months without current sign of recurrence; 3) at least 3 months after adjuvant treatment (radiation, chemotherapy); 4) suffered shoulder/axillary pain that began after the breast cancer surgery; 5) diagnosis of MPS in the internal rotator muscle (subscapularis and/or pectoralis) of the shoulder. Exclusion criteria included: 1) bilateral breast cancer; 2) MPS in other muscles than the subscapularis or pectoralis muscle; 3) received other therapeutic modalities such as analgesic, physical therapies or different types of injections during study periods; 4) neurologic shoulder/axillary pain, if the patient had a history of posterior neck pain; 5) signs and symptoms of neuropathy in the upper limbs; and 6) a 0-10 range visual analogue scale (VAS) score <5. Diagnosis of MPS was based on the modified criteria described by Simons10 The criteria were: 1) tender spots in the subscapularis or pectoralis muscle; 2) typical pattern of referred pain elicited when tender spots are compressed; 3) restricted ROM; and 4) LTRs during US-guided trigger point injections.11
This study was approved by the Institutional Review Board and human subjects review committee before the study began. Written informed consent was obtained from all participants after they were briefed on the purpose and procedures of the study.
All patients had a diagnosis of at least one active MTrP in the subscapularis and/or pectoralis muscle based on the previously outlined criteria. US-guided trigger point injection methods have been previously reported for lower back muscles and were modified for MTrPs in the subscapularis or pectoralis.8,11
We performed B-mode, real-time ultrasonography with sterile coupling gel and a latex-free transducer cover using an Accuvix XG US machine (Medison Co., Seoul, Korea) interfaced with a 5- to 12-MHz linear array transducer around the targeted muscle. A physiatrist (S.C.L) with more than 8 years of experience in musculoskeletal ultrasonography carried out US-guided injection procedures.
For procedures involving the pectoralis muscle, the patient lay supine with the arm at side and the hand supinated. For procedures involving the subscapularis muscle, the patient lay supine with the shoulder abducted, elbow flexed, hand supinated, and scapula rotated as upwardly as possible to expose the subscapularis (Fig. 1). In this position, only the teres major muscle was located above the subscapularis. The pectoralis major and latissimus dorsi were not placed in the treatment window.
The region was scanned and a transverse plane was obtained to visualize the target muscle with tender spots in the subscapularis or pectoralis muscle. In case of multiple tender spots in one muscle, US scanning and injection procedures were repeated for all tender spots. In contrast to the pectoralis muscle, the depth of the subscapularis muscle necessitated localization using ultrasonography (Fig. 2). First, the lateral border of the scapula was identified by ultrasonography (Fig. 3). As the subscapularis was visualized on US, the probe traced sites for trigger point injection (Fig. 4). A color Doppler images were used to avoid the neurovascular bundle. The Doppler setting changed at the level where vascular structures were optimally visualized in each subject. Under US-guidance, a 25-gauge, 3.8-cm needle connected to a 5-mL syringe containing 0.5% lidocaine was inserted into the pectoralis at the MTrPs region, and a 23-gauge, 6.0-cm needle connected to a 5-mL syringe was used for the subscapularis muscles. A physiatrist observed LTRs on ultrasonography while performing trigger point injections (Supplementary video 1 and 2). With the use of a short axis view of the needle tip (coaxial method), the needle passing through the skin and adipose tissue to penetrate the muscle was visualized. Repeated needling was performed to different loci in that region to elicit as many LTRs as possible. If no LTR was observed after 8 to 10 attempts, needling was stopped. At that point, a drop of 0.5% lidocaine was injected to reduce post-needling soreness. The injection site was pressed to ensure proper homeostasis after the procedure.
US-guided trigger point injections were performed in all affected muscles at 1-week intervals. Additional injections were not considered if patients were satisfied with the reduction in discomfort or pain severity, or if the patient did not want another injection for other reasons. No other therapies, such as physical therapy or medications, were allowed during the study period, so as not to affect the results. However, self-exercise and behavior correction were allowed to avoid early recurrence of pain after trigger point injections. The patients were taught to do self-exercise comprising stretching exercise (repeated 20 times during a day) to alleviate MTrPs and to avoid the posture that might aggravate the symptoms.
The primary outcome measures were pain intensity during the passive external rotation of the affected shoulder. Pain intensity was described by the patient on a VAS of 0-10, for which 0 signified no pain and 10 signified the most severe pain ever experienced. Secondary outcome measures were the passive ROM of the shoulder for external rotation and abduction using goniometry while in a seated position. In addition, we reviewed and monitored any side effects of trigger point injections throughout the study. Outcome measurements were made at baseline, immediately after the first injection and 3 months after the last injection by a clinical researcher who was not blinded to the study.
Demographic data were collected on all patients including age, body mass index, information regarding breast cancer, and medical history. In addition, data were collected regarding the type of surgery performed, type of lymph node dissection, use of adjuvant treatment, lymphedema, and rotator cuff tear.
SPSS version 20.0 software (SPSS Inc, Chicago, IL, USA) was used for the statistical analyses. Shapiro-Wilk test was used for all continuous variables for determining whether or not the distribution was normal. The results of VAS and ROM of the shoulder for external rotation and of abduction did not show normal distribution (p<0.05 by the Shapiro-Wilk test). Therefore, the Wilcoxon signed rank test with Bonferroni correctionwas used to analysis the effect of trigger point injection.
All participants were divided into good responder who showed 4 or below 4 and bad responder who showed more than 4 in immediate VAS after the first trigger point injection. Mann-Whitney U test or chi-square test was used to compare demographic and clinical characteristics between the good and bad responder group. For the analysis, the types of surgery were grouped into mastectomy (modified radical mastectomy and nipple-areola complex sparing total mastectomy) and partial mastectomy (breast conserving surgery). Statical significance was assumed to be at p<0.05.
Between April 2011 and June 2012, 78 women with breast cancer who underwent surgery were referred to a physiatrist (S.C.L) for shoulder/axillary pain. Of these, nineteen women met the eligibility criteria. Follow-up evaluation at 3 months after the last trigger point injection was available for all 19 patients (age range, 30-76 years). Demographic and clinical characteristics of patients are summarized (Table 1). The average±SD of the number of trigger point injections was 2.7±1.3. The subscapularis muscle was affected in all 19 patients and the pectoralis muscle was affected in six of the 19 patients. In all affected muscles, LTRs were observed during US-guided trigger point injections. No patient was affected only in the pectoralis muscle. At baseline, in patients in whom MTrPs existed simultaneously in the pectoralis and subscapularis muscles, pain severity was greater and ROM of shoulder external rotation and abduction were more restricted, compared to patients with MTrPs in the subscapularis muscle only (p<0.05). However, responses to treatment were not very different.
VAS and ROM of the shoulder for external rotation and of abduction showed significant improvement immediately after the first injection and at 3 months after the last injection compared with baseline (p<0.05) (Table 2). Treatment effects decreased as treatment was delayed, initial pain severity was high, and limitation of ROM of the shoulder was severe (p<0.05). Patients did not report any complications related to the procedure or serious adverse events attributable to the treatment. There were no infections or vascular injuries.
There was no significant difference in demographic and clinical characteristics including cancer stage, method of operation and history of adjuvant therapy between the good and the bad responder group. However, the duration from onset to surgery and the duration of MPS in the good responder group were significantly shorter than the bad responder group (p<0.05).
To our best knowledge, this is the first report of US-guided trigger point injections into MTrPs in the subscapularis and pectoralis muscles, in post mastectomy patients. Treatment with a trigger point injection of the subscapularis and/or pectoralis muscles experienced relief of the symptoms in 74% of the patients after the first trigger point injection, regardless of other associated factors. As we performed trigger point injections under US-guidance and observed LTRs (one of the objective signs of MTrPs), we could exclude the possibility of an injection outside the targeted muscle leading to a satisfactory response. In case of MTrPs in the subscapularis and/or the pectoralis major after breast cancer surgery, treatment effects decreased as treatment was delayed. Therefore, early intervention is required for MTrP in the internal rotators of the shoulder.
Hamada, et al.12 documented a case series of 27 patients with post-thoracotomy pain, which is often assumed to be neuropathic in origin, and found the primary source of pain to be MTrPs in 67% of the patients. The authors commented that the existence of MTrPs significantly increased the rate of success after treatment. In a cohort of 163 breast cancer patients, 21% also showed active MTrPs.13 One case report described the activation of the pectoralis major MTrPs as a result of muscle trauma after transaxillary surgery.14 Given the fact that breast cancer treatment directly involves the neuromusculoskeletal tissues of one or more shoulder girdle regions, it is not surprising that shoulder pain, limited motion, and weakness are frequently reported among breast cancer survivors.15,16,17 Breast cancer survivors in a post-operative state often display restricted movement of the shoulder on the affected side due to pain, tightness related to surgery or radiation, and a general protective tendency resulting from fear or anxiety.18,19
Active MTrPs in the pectoralis muscle are most prevalent in breast cancer groups.7 As the pectoralis muscle is anatomically located directly deep to the breast, it is possible that it would be the most affected muscle by the surgical procedure. However, no studies on MTrPs in the subscapularis of post-mastectomy patients are found in the literature. The subscapularis muscle, the largest of the rotator cuff muscles, acts as a shoulder internal rotator and originates from the entire subscapular fossa and inserts onto the lesser tuberosity of the humerus. Muscle tension of the subscapularis resulting from immobilization may overwhelm the other rotator cuff muscles that are relatively weak. As a result, internal rotation and adduction contracture of the shoulder joint with associated pain occurs frequently.20,21 This can lead to reduced external rotation and insufficient acromial elevation, provoking rotator cuff tendon impingements.22 MTrPs in the subscapularis often result from, or are perpetuated by, acute or chronic overuse of the muscle, trauma, prolonged immobilization (e.g., when the arm is held internally rotated in a sling or cast), and chronic shortening of the muscle (e.g., when the patient has chronic rounded shoulders posture with arms internally rotated).9 As MTrPs in the subscapularis tend to produce restricted and painful external rotation of the arm at the shoulder joint (because external rotation of the arm is required for full abduction of the arm, arm abduction is also often restricted), MTrPs in the subscapularis are often incorrectly assessed as frozen shoulder, rotator cuff lesions, cervical disc syndrome, or thoracic outlet syndrome.9
Since the subscapularis is located deep between the scapula and the rib cage, diagnosis and treatment of MTrPs in the subscapularis muscle are difficult. In the present study, we found multiple cases of active MTrPs in the subscapularis muscle of patients with breast cancer using the novel US-guided method. The new US-guided technique will provide a good safe and accurate approach for diagnosis and treatment of MTrPs in the subscapularis muscle.
Ultrasonographic machines are inexpensive, portable, and readily accessible as they continue to become standard equipment in most hospitals. Ultrasonography is a reliable tool that allows for real-time scanning of the targeted structure, and is useful for detecting LTRs in MTrPs in deeply located muscles.11 In addition, US-guidance could facilitate depth control during injection for even the deep and less accessible MTrPs in the subscapularis and reduce potential inadvertent injuries that could be caused by improper needle placement.
There is a case report on blind trigger point injections of the subscapularis muscle in patients with diseases other than breast cancer,23 and there is also a study to describe an US-guided lateral approach for the injection of spastic subscapularis muscle.8 However, we feel that our new US-guided trigger point injection would be advantageous over the older methods, by allowing safe and effective injection of MTrPs in the subscapularis muscle.
There are several limitation of our study, including selection bias, no control group, and the short-term follow-up of a small number of patients. Furthermore, we did not find a relationship between MTrPs and demographic and clinical data such as the type of surgery, type of lymph node dissection, and use of adjuvant treatments, which are potentially important in the development of MTrPs. The present success in relieving shoulder pain by trigger point injections need to be confirmed with a controlled and long-term studies involving more patients. Although there were no patients in this study who had MTrPs exclusively in the pectoralis, not in the subscapularis, our patients group might not reflect all those who have had surgery for with breast cancer. This study did not address the prevalence of MTrPs in patients with breast cancer. Previous studies on the prevalence of MTrPs in patients with breast cancer did not mentioned MTrPs in the subscapularis muscle. This is not because of a lack of MTrPs in the subscapularis muscle, but it is more likely due to a lack of proper methods for the diagnosis and treatment of the subscapularis MTrPs. In addition, we did not exclude the patients with adhesive capsulitis. Although all participants' symptoms satisfied MPS criteria, there was some overlapping of symptoms which are suggestive of both adhesive capsulitis and MPS in shoulder internal rotators. Ironically, however, MPS occurs less frequently as a consequence of primary muscle lesion.24,25,26 Considerable evidence exists to suggest that MPS is frequently caused by or related to a lesion in another soft tissue such as adhesive capsulitis.26
In conclusion, the current study shows the existence of MTrPs in the subscapularis as well as in the pectoralis muscle in women who had received breast cancer surgery. In breast cancer patients with shoulder pain, US-guided trigger point injections of the subscapularis or pectoralis are effective for both diagnosis and treatment when the cause of shoulder pain is suspected to originate from MTrPs in these muscles, particularly, the subscapularis. Therefore, we recommend that MPS should be considered in post-mastectomy patients with shoulder pain. Furthermore, since treatment effects decreased when treatment was delayed, initial pain severity was high, and limited motion of the shoulder was severe for MTrPs of the subscapularis or pectoralis muscle in the post-mastectomy state, therefore, early intervention is recommended when MTrPs are suspected in these muscles.
Figures and Tables
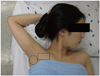 | Fig. 1Patient position for the subscapularis. The patient lay supine with the shoulder abducted, elbow flexed, hand supinated, and the scapula rotated as upwardly as possible to move the inferior angle away from the midline. The circles indicate the area of ultrasound examination for trigger point injection of the subscapualris compared with the square for the pectoralis muscle. |
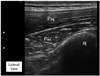 | Fig. 2A trasverse plane ultrasound image of the pectoralis muscle. This superficial muscle was easily detected on ultrasound image. Pmj, pectoralis major; Pmi, pectoralis minor; R, rib. |
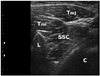 | Fig. 3A trasverse plane ultrasound image of the subscapularis muscle. Before the trigger point injection, the lateral border of the scapula needs to be identified. C chest wall, L lateral border of the scapula. Tmj, Teres major; Tmi, Teres minor; SSC, subscapularis. |
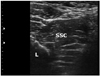 | Fig. 4The transducer of ultrasound (US) was moved from lateral to medial. Then, the subscapularis muscle needed to be optimally visualized under US-guidance to perform trigger point injections. L, lateral border of the scapula; SSC, subscapularis. |
Table 2
Changes in Visual Analog Scale (VAS) and Range of Motion (ROM) before and after Treatment

ROMER, external rotation of ROM; ROMAb, abduction of ROM; (1), measures at baseline; (2), measures immediate after the first injection; (3) measures 3 months after the last injection.
The range of the VAS was 0-10 with 0 meaning no pain and 10 meaning maximal pain, and also that the units of the ROM are in degrees. VAS and ROM of the shoulder for external rotation and of abduction showed significant improvement immediate after the first injection and at 3 months after the last injection compared with baseline, respectively (*p<0.05).
References
1. Lee SA, Kang JY, Kim YD, An AR, Kim SW, Kim YS, et al. Effects of a scapula-oriented shoulder exercise programme on upper limb dysfunction in breast cancer survivors: a randomized controlled pilot trial. Clin Rehabil. 2010; 24:600–613.

2. Ebaugh D, Spinelli B, Schmitz KH. Shoulder impairments and their association with symptomatic rotator cuff disease in breast cancer survivors. Med Hypotheses. 2011; 77:481–487.

3. Cheville AL, Tchou J. Barriers to rehabilitation following surgery for primary breast cancer. J Surg Oncol. 2007; 95:409–418.

4. Stubblefield MD, Custodio CM. Upper-extremity pain disorders in breast cancer. Arch Phys Med Rehabil. 2006; 87:3 Suppl 1. S96–S99.

5. Torres Lacomba M, Mayoral del Moral O, Coperias Zazo JL, Gerwin RD, Goñí AZ. Incidence of myofascial pain syndrome in breast cancer surgery: a prospective study. Clin J Pain. 2010; 26:320–325.

6. Simons DG. Review of enigmatic MTrPs as a common cause of enigmatic musculoskeletal pain and dysfunction. J Electromyogr Kinesiol. 2004; 14:95–107.

7. Fernández-Lao C, Cantarero-Villanueva I, Fernández-de-Las-Peñas C, Del-Moral-Ávila R, Menjón-Beltrán S, Arroyo-Morales M. Development of active myofascial trigger points in neck and shoulder musculature is similar after lumpectomy or mastectomy surgery for breast cancer. J Bodyw Mov Ther. 2012; 16:183–190.

8. Rha DW, Han SH, Kim HJ, Won SY, Lee SC. Ultrasound-guided lateral approach for needle insertion into the subscapularis for treatment of spasticity. Arch Phys Med Rehabil. 2012; 93:1147–1152.

9. Muscolino JE. The muscle and bone palpation manual with trigger points, referral patterns, and stretching. 1st ed. St. Louis (Mo): Mosby Inc;2009.
10. Simons DG. Muscle pain syndromes--Part I. Am J Phys Med. 1975; 54:289–311.
11. Rha DW, Shin JC, Kim YK, Jung JH, Kim YU, Lee SC. Detecting local twitch responses of myofascial trigger points in the lower-back muscles using ultrasonography. Arch Phys Med Rehabil. 2011; 92:1576–1580.

12. Hamada H, Moriwaki K, Shiroyama K, Tanaka H, Kawamoto M, Yuge O. Myofascial pain in patients with postthoracotomy pain syndrome. Reg Anesth Pain Med. 2000; 25:302–305.

13. Cheville AL, Troxel AB, Basford JR, Kornblith AB. Prevalence and treatment patterns of physical impairments in patients with metastatic breast cancer. J Clin Oncol. 2008; 26:2621–2629.

14. Cummings M. Myofascial pain from pectoralis major following trans-axillary surgery. Acupunct Med. 2003; 21:105–107.

15. Lee TS, Kilbreath SL, Refshauge KM, Herbert RD, Beith JM. Prognosis of the upper limb following surgery and radiation for breast cancer. Breast Cancer Res Treat. 2008; 110:19–37.

16. Levangie PK, Drouin J. Magnitude of late effects of breast cancer treatments on shoulder function: a systematic review. Breast Cancer Res Treat. 2009; 116:1–15.

17. Gyedu A, Kepenekci I, Alic B, Akyar S. Evaluation of muscle atrophy after axillary lymph node dissection. Acta Chir Belg. 2009; 109:209–215.

18. Kim SM, Park JM. Normal and abnormal US findings at the mastectomy site. Radiographics. 2004; 24:357–365.

19. Katz J, Poleshuck EL, Andrus CH, Hogan LA, Jung BF, Kulick DI, et al. Risk factors for acute pain and its persistence following breast cancer surgery. Pain. 2005; 119:16–25.

20. Harrison TP, Sadnicka A, Eastwood DM. Motor points for the neuromuscular blockade of the subscapularis muscle. Arch Phys Med Rehabil. 2007; 88:295–297.

21. Yelnik AP, Colle FM, Bonan IV. Treatment of pain and limited movement of the shoulder in hemiplegic patients with botulinum toxin a in the subscapular muscle. Eur Neurol. 2003; 50:91–93.

22. Salisbury SK, Choy NL, Nitz J. Shoulder pain, range of motion, and functional motor skills after acute tetraplegia. Arch Phys Med Rehabil. 2003; 84:1480–1485.

23. Ingber RS. Shoulder impingement in tennis/racquetball players treated with subscapularis myofascial treatments. Arch Phys Med Rehabil. 2000; 81:679–682.

24. Chou LW, Hsieh YL, Chen HS, Hong CZ, Kao MJ, Han TI. Remote therapeutic effectiveness of acupuncture in treating myofascial trigger point of the upper trapezius muscle. Am J Phys Med Rehabil. 2011; 90:1036–1049.

25. Hong CZ. Pathophysiology of myofascial trigger point. J Formos Med Assoc. 1996; 95:93–104.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download