Abstract
Purpose
To examine the effects of conservative and surgical treatments for nocturnal leg cramps in patients with lumbar spinal stenosis (LSS). Nocturnal leg cramps is frequently observed in patients with peripheral neuropathy. However, there have been few reports on the relationship between nocturnal leg cramps and LSS, and it remains unknown whether conservative or surgical intervention has an impact on leg cramps in patients with LSS.
Materials and Methods
The subjects were 130 LSS patients with low back and leg pain. Conservative treatment such as exercise, medication, and epidural block was used in 66 patients and surgical treatment such as decompression or decompression and fusion was performed in 64 patients. Pain scores and frequency of nocturnal leg cramps were evaluated based on self-reported questionnaires completed before and 3 months after treatment.
Results
The severity of low back and leg pain was higher and the incidence of nocturnal leg cramps was significantly higher before treatment in the surgically treated group compared with the conservatively treated group. Pain scores improved in both groups after the intervention. The incidence of nocturnal leg cramps was significantly improved by surgical treatment (p=0.027), but not by conservative treatment (p=0.122).
Conclusion
The findings of this prospective study indicate that the prevalence of nocturnal leg cramps is associated with LSS and severity of symptoms. Pain symptoms were improved by conservative or surgical treatment, but only surgery improved nocturnal leg cramps in patients with LSS. Thus, these results indicate that the prevalence of nocturnal leg cramps is associated with spinal nerve compression by LSS.
Compression of the spinal nerve roots by lumbar spinal stenosis (LSS) is a major clinical problem associated with intermittent claudication, pain, numbness, and lack of normal sensitivity. Such compression has been shown to induce neurophysiologic dysfunction, degeneration, and reduced blood flow in nerve roots in animal models and in humans.1,2 In a survey of outpatients, 56% reported nocturnal leg cramps and 49% reported symptoms of peripheral neuropathy.3 Conditions that were especially related to leg symptoms included hypertension, peripheral vascular disease, coronary artery disease, cerebrovascular disease, kidney disease, and hypokalemia.3 In particular, nerve root compression and peripheral vascular disease are thought to be predisposing factors for nocturnal leg crampss.4
There have been few reports on the relationship between leg cramps and LSS, and it is unknown whether conservative or surgical intervention has an impact on leg cramps in patients with LSS. In an evaluation of the prevalence of leg cramps in patients with LSS treated surgically and the relationship between leg cramps and surgical outcomes, based on results obtained from a self-completed questionnaire sent by mail at a mean of 3.6 years after decompression surgery, LSS patients had significantly more frequent attacks of nocturnal leg cramps than a control population, and leg cramps disturbed quality of life and rarely improved after decompression surgery.5
Ankle-brachial index (ABI), an estimation of blood flow in the main artery in the leg, before and 3 months after conservative or surgical treatment of patients with LSS has been prospectively reported, and ABI significantly increased after either type of treatment was found.6 This result suggests that both conservative and surgical treatment can improve nocturnal leg cramps in patients with LSS. Therefore, the purpose of the current study was to investigate the effects of these therapies on the incidence of nocturnal leg cramps symptoms in patients with LSS.
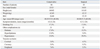
The subjects were 130 patients with low back and leg pain that had continued for at least 1 month. Patients who had previously undergone spinal surgery were excluded from the study. We also excluded patients with spinal tumor, infection, or trauma. LSS was diagnosed on X-ray and magnetic resonance imaging (MRI) and by physical examination by spine surgeons. On the MRI, the degree of spinal stenosis varied from slight to severe, and central stenosis, stenosis of the lateral recess, and foraminal stenosis were apparent. Patients with monoradiculopathy, polyradiculopathies, or cauda equine syndrome were included in the study. The background of the subjects is shown in Table 1. The ethics committee of our institution approved the protocol for the human procedures used in this study. Informed written consent was obtained from each subject.
The patients were divided into groups that received conservative or surgical treatment. Conservative treatment was applied in patients who did not receive any therapy before visiting a hospital. Surgery was used for patients in whom previous conservative treatment was ineffective. Therefore, seriously impaired patients underwent surgery and those with less impairment received conservative care.
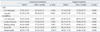
Patients in the conservative group underwent treatment including exercise (walking, walking in a pool, muscle training, and muscle stretching), medication, and epidural block. Walking and walking in a pool were performed by the patients without the help of a therapist. Muscle training and stretching were performed for the abdominal and lower extremities with the assistance of physical therapists. Medication included non-steroidal anti-inflammatory drugs (NSAIDs), vitamins, muscle relaxants, and prostaglandin E1 (PGE1), as determined by the personal physician for each patient. A transforaminal or caudal epidural block was administered in some patients, and exercise, drugs, and blocks were used in some cases. The details are shown in Table 2.
In the surgery group, the patients had undergone exercise therapy, medication, and epidural block before surgery. After surgery, the patients also received exercise therapy and medication, but were not given an epidural block. The percentages of patients who received exercise therapy and medication before and after surgery are shown in Table 2.
The JOA Back Pain Evaluation Questionnaire (JOABPEQ: including low back pain, lumbar function, walking ability, social life, and mental health) and a visual analogue scale (VAS: from 0 to 100, 100: worst) were evaluated for each patient. The range of the JOABPEQ score for each domain is 0 to 100, with higher scores indicating a better condition. The five functional scores are used independently. Low back pain and leg pain were evaluated before and 3 months after treatment.
The background of the 130 patients in the study is shown in Table 1. Age ranged from 40 to 80 years old, with average ages of 68.45±9.20 and 68.00±9.13 years old in the conservative and surgical groups, respectively (mean±SD). Among the patients, 17.3% in the conservative group and 17.0% in the surgical group were smokers. Complications included diabetes, hyperlipidemia, hypertension, and vascular occlusion (cerebral, coronary, and peripheral) in both groups. The details are shown in Table 1.
Treatment given over 3 months in the two groups is shown in Table 2. The patients in the conservative group received exercise therapy (40%), medications (100%), and epidural block (18%). PGE1 was the most common drug (85.0%) and NSAIDs, vitamins, and muscle relaxants were also used. The patients in the surgical group also received exercise therapy (45%) and medication (100%) before and in the 3 months after surgery. Epidural block (18%) was given only before surgery. Fifty patients underwent decompression surgery and 14 underwent decompression and posterior fusion surgery. There were no perioperative complications. In the conservative group, no patients underwent surgery for severity of symptoms during the 3-month study period.
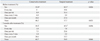
Pains scores in the two groups are shown in Table 3. VAS scores for low back pain in the conservative group were significantly lower than those in the surgical group (p<0.05). JOABPEQ scores in 4 categories (low back pain, lumbar function, walking ability, and social life) in the conservative group were also significantly better than those in the surgical group (p<0.05). After conservative treatment for 3 months, there were significant improvements in VAS scores for low back pain and leg pain (p<0.05) and in JOABPEQ scores in all five categories (the four listed above and mental health, p<0.01) (Table 4). VAS scores for low back pain and leg pain (p<0.001) and JOABPEQ scores in all categories were also significantly improved at 3 months after surgery (p<0.001) (Table 4).
Before treatment, nocturnal leg cramps was present in 52.8% of the patients in the conservative group and in 82.3% in the surgical group (Table 5), with a significant difference in incidence between the groups (p=0.026). After 3 months of treatment, nocturnal leg cramps persisted in 39.3% and 45.1% of patients in the respective groups, with no significant difference in incidence between the groups (p=0.056). The incidence of nocturnal was not improved significantly by conservative treatment leg cramps was improved significantly by surgery (p=0.027), but (p=0.122).
In the current study, both conservative and surgical treatment improved low back pain and leg pain originating from lumbar spinal stenosis. Before treatment, the severity of low back and leg pain was higher in patients treated surgically compared to those who received conservative treatment, and the incidence of nocturnal leg cramps was also significantly higher in patients who underwent surgery. Pain symptoms improved in both groups of patients after treatment and the incidence of nocturnal leg cramps was improved significantly by surgery, but not by conservative treatment. These findings indicate that the prevalence of nocturnal leg cramps is associated with spinal nerve compression and with the severity of symptoms in patients with LSS.
Nerve root compression and peripheral vascular disease are thought to be predisposing factors for nocturnal leg cramps,4 but there are only a few studies on the association between leg cramps and lumbar spinal diseases. Rish suggested that leg cramps is common in patients with lumbar radiculopathy7 and neurological diseases are significantly more common in patients with nocturnal leg cramps (36%) than in controls (18%).8 Demircan, et al.9 reported positive cramp findings preoperatively in 133 patients (72%) who underwent surgery and a positive straight leg raising test in all patients with lumbar disc herniation. In an evaluation of the prevalence of leg cramps in patients with LSS treated surgically, Matsumoto, et al.5 found a relationship between leg cramps and surgical outcomes and showed that the patients had significantly more frequent nocturnal leg cramps compared to a control population. In the current study, patients who underwent surgery had more severe symptoms and a higher incidence of nocturnal leg cramps compared with those who received conservative treatment. Thus, we suggest that the severity of nerve root compression may be associated with nocturnal leg cramps in patients with LSS.
Non-pharmacologic treatment such as walking and stretching is recommended for nocturnal leg cramps.10 However, a recent controlled study showed that calf stretching was ineffective for reducing the occurrence of nocturnal leg cramps.11 Several randomized, double-blind, placebo-controlled studies have been conducted to assess the efficacy and safety of drugs for leg cramps, including quinine sulfate, magnesium sulfate, verapamil, diltiazem, vitamin E, vitamin B complex, naftidrofuryl oxalate, orphenadrine citrate, gabapentin, lidocaine, and botulinum toxin.4 However, these studies have yielded mixed and controversial results regarding the efficacy of these drugs.4 In the current study, the patients with LSS who were treated conservatively used non-pharmacologic treatment such as walking and stretching and drugs such as NSAIDs, vitamins, muscle relaxants, and prostaglandin E1 showed no significant improvement in nocturnal leg cramps.
There have also been few studies of the efficacy of surgical treatment for nocturnal leg cramps originating from lumbar disease. A retrospective study of the prevalence of leg cramps in patients with LSS treated surgically suggested that nocturnal leg cramps did not improve after decompression surgery;5 however, these findings were based on a self-reported questionnaire answered by mail at 3.6 years after surgery. In contrast, cramp findings were positive in 70%, 52%, 34%, and 8% of patients in postoperative months 1, 3, 12, and 24, respectively, in patients with lumbar disc herniation,9 suggesting that surgical decompression was effective for reducing the frequency of nocturnal leg cramps. In the current prospective study, symptoms such as low back pain and leg pain were improved by conservative or surgical treatment; however, the incidence of nocturnal leg cramps was significantly improved only by surgical treatment after 3 months. Therefore, we concluded that surgical decompression for spinal nerve roots was itself effective for improvement of nocturnal leg cramps in LSS patients, and that compression of spinal nerve roots is closely associated with symptoms of nocturnal leg cramps in these patients.
There are several limitations in the current study. First, it is a relatively small study with a restricted number of patients. Second, the study was not randomized: that is, seriously impaired patients underwent surgery while those who were less impaired received conservative care. Third, we did not evaluate any relationship between stenosis level(s) or severity and leg cramps, and incidence of nocturnal leg cramps between single stenosis and multiple stenosis. Finally, we did not show effective level for decompression surgery to improve nocturnal leg cramps, and risk factors for advanced leg cramps in the conservative group. Further investigation is required to clarify these points.
Within these limitations, we conclude that the frequency of nocturnal leg cramps in patients with lumbar spinal stenosis before treatment is likely to be significantly higher in those with severe pain compared with those with less pain. The incidence of nocturnal leg cramps can be significantly improved by surgery, but not by conservative treatment.
Figures and Tables
References
1. Konno S, Yabuki S, Sato K, Olmarker K, Kikuchi S. A model for acute, chronic, and delayed graded compression of the dog cauda equina. Presentation of the gross, microscopic, and vascular anatomy of the dog cauda equina and accuracy in pressure transmission of the compression model. Spine (Phila Pa 1976). 1995; 20:2758–2764.

2. Sekiguchi M, Kikuchi S, Myers RR. Experimental spinal stenosis: relationship between degree of cauda equina compression, neuropathology, and pain. Spine (Phila Pa 1976). 2004; 29:1105–1111.
3. Oboler SK, Prochazka AV, Meyer TJ. Leg symptoms in outpatient veterans. West J Med. 1991; 155:256–259.
5. Matsumoto M, Watanabe K, Tsuji T, Ishii K, Takaishi H, Nakamura M, et al. Nocturnal leg cramps: a common complaint in patients with lumbar spinal canal stenosis. Spine (Phila Pa 1976). 2009; 34:E189–E194.
6. Ohtori S, Yamashita M, Murata Y, Eguchi Y, Aoki Y, Ataka H, et al. Conservative and surgical treatment improves pain and ankle-brachial index in patients with lumbar spinal stenosis. Yonsei Med J. 2013; 54:999–1005.

8. Haskell SG, Fiebach NH. Clinical epidemiology of nocturnal leg cramps in male veterans. Am J Med Sci. 1997; 313:210–214.

9. Demircan MN, Colak A, Kutlay M, Kibici K, Topuz K. Cramp finding: can it be used as a new diagnostic and prognostic factor in lumbar disc surgery? Eur Spine J. 2002; 11:47–51.

10. Man-Son-Hing M, Wells G. Meta-analysis of efficacy of quinine for treatment of nocturnal leg cramps in elderly people. BMJ. 1995; 310:13–17.

11. Coppin RJ, Wicke DM, Little PS. Managing nocturnal leg cramps--calf-stretching exercises and cessation of quinine treatment: a factorial randomised controlled trial. Br J Gen Pract. 2005; 55:186–191.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download