Abstract
Purpose
The elucidation of thalamocortical connections between the mediodorsal nucleus (MD) of thalamus and the prefrontal cortex (PFC) is important in the clinical fields of neurorehabilitation and psychiatry. However, little is known about these connections in human brain. We attempted to identify and investigate the anatomical characteristics of the thalamocortical connection between MD and PFC in human brain using diffusion tensor tractography (DTT).
Materials and Methods
Thirty-two healthy volunteers were recruited for this study. Diffusion tensor images were scanned using a 1.5-T. A seed region of interest was placed at the MD of the thalamus on coronal images, and target regions of interest were placed on the dorsolateral prefrontal cortex (DLPFC), the ventrolateral prefrontal cortex (VLPFC), and the orbitofrontal cortex (OFC), respectively. The three thalamocortical connections found were reconstructed using Functional Magnetic Resonance Imaging of the Brain (FMRIB) software.
Results
The three thalamocortical connections were arranged in subcortical white matter in the following order from upper to lower levels: the DLPFC, the VLPFC, and the OFC. In terms of fractional anisotropy and mean diffusivity values, no significant differences were observed between the DLPFC, VLPFC and OFC (p>0.05). In contrast, the OFC tract volume was higher than those of the DLPFC and the VLPFC (p<0.05).
The prefrontal cortex (PFC) is involved in numerous cognitive functions, such as working memory, attention, arousal, decision making, execution, behavior inhibition, and motivation.1,2 Each subregion of the PFC has a specific role in cognition. The main functions of the four subregions of the PFC are as follows: dorsolateral prefrontal cortex (DLPFC)-working memory, ventrolateral prefrontal cortex (VLPFC)-deliberation of decision making and goal-directed behavior, the orbitofrontal cortex (OFC)-emotional control and inhibitory control of behavior, and the medial PFC-motivation and initiation of activity.1,2,3,4,5 These PFCs receive copious afferent fibers from the mediodorsal nucleus (MD) of the thalamus via anterior thalamic radiation.6
Injury of the PFC can cause frontal syndrome, which includes cognitive and emotional dysfunctions.1 On the other hand, patients with a lesion in the MD of the thalamus can exhibit clinical manifestations similar to those associated with a PFC injury, possibly because of thalamocortical connections between the MD of the thalamus and the PFC.7,8,9 Furthermore, frontal network syndrome has also been referred to patients with a lesion within the thalamocortical connections between the MD of the thalamus and the PFC.1 Furthermore, these connections are also known to be involved in many psychiatric diseases, such as, schizophrenia, addiction, depression, and bipolar disorder.2,10 Therefore, the elucidation of these thalamocortical connections would undoubtedly be useful in the clinical field in terms of neurorehabilitation and psychiatry. Many animal studies have demonstrated these thalamocortical connections,6,11,12,13,14,15,16,17 however, detailed neural pathways of these connections in human brain have not yet been identified.18,19
The recent development of diffusion tensor tractography (DTT), which was derived from diffusion tensor imaging (DTI), allows visualization and localization of neural tracts at the subcortical level in three dimensions. DTT enables the state of thalamic radiations and thalamus-related neural tracts to be assessed in human brain.20,21,22,23,24,25 Nevertheless, little is known about the thalamocortical connections between the MD of thalamus and the PFC in human brain.18,19
The present study was undertaken to identify and investigate the anatomical characteristics of thalamocortical connections between the MD and the PFC in human brain using DTT.
Thirty-two normal healthy subjects (19 males and 13 females of mean age 36.94±11.51 years; range 20 to 62) with no history of neurologic disease were recruited for this study. All participants provided written consent, and the study was approved by the Institutional Review Board of our university hospital.
DTI data were acquired using a 6-channel head coil on a 1.5 T Philips Gyroscan Intera (Philips, Best, the Netherlands) and single-shot echo-planar imaging. For each of the 32 non-collinear diffusion sensitizing gradients, we acquired 67 contiguous slices parallel to the anterior commissure-posterior commissure line. Imaging parameters were as follows: acquisition matrix=96×96; reconstructed matrix=192×192; field of view=240×240 mm2; TR=10726 ms; TE=76 ms; parallel imaging reduction factor (SENSE factor)=2; EPI factor=49; b=1000 s/mm2; NEX=1; and a slice thickness of 2.5 mm with no gap.
Diffusion-weighted imaging data were analyzed using software from the Oxford Centre for Functional Magnetic Resonance Imaging of the Brain (FMRIB) Software Library (FSL v5.0; www.fmrib.ox.ac.uk/fsl). Affine multi-scale two-dimensional registration was used for correction of head motion and eddy current-induced image distortion. Fiber tracking was performed using a probabilistic tractography method based on a multiple tensor model, using tractography routines implemented in FMRIB Diffusion (5000 streamline samples, 0.5 mm step lengths, curvature thresholds=0.2).26
In each case, a seed region of interest (ROI) was placed at the MD of the thalamus on a coronal image of b0 image (no diffusion weighting applied). MD location was defined as previously described.19,27 For thalamocortical connections from the MD to the DLPFC and VLPFC, we defined the DLPFC as Brodmann areas (BAs) 8, 9, and 46, and the VLPFC as BAs 44, 45, and 47, and manually drew target ROIs on the DLPFC and VLPFC on the coronal images, respectively (Fig. 1).19,28 For thalamocortical connection from the MD to the OFC, we defined the OFC as BAs 47/12, 10, 11, and 13, and manually drew the target ROI on the OFC on an axial image.19,29 Thalamocortical connections between the MD and the PFC were determined by selecting fibers passing through seed and target ROIs. Of 5000 samples generated from the seed voxel, contact results were visualized at a threshold minimum of 1 streamline through each voxel for analysis. Fractional anisotropy (FA), mean diffusivity, and tract volume in the three thalamocortical connections to each PFC were then measured.
SPSS software (v.15.0; SPSS, Chicago, IL, USA) was used for the data analysis. One-way analysis of variance (ANOVA) with Tukey's post-hoc test was used to determine significances of difference in DTI parameters between reconstructed thalamocortical connections. Statistical significance was accepted for p values of <0.05.
The three reconstructed thalamocortical connections between the thalamic MD and the PFC are shown in Fig. 1. These thalamocortical connections were arranged in subcortical white matter in the following order; DLPFC, VLPFC, and OFC. Although some individual variations were observed, the main courses of these three thalamocortical connections were as follows; thalamocortical connections to the DLPFC ascended through the anterior thalamic radiation via the anterior limb and genu of the upper internal capsule, the corona radiata around the anterior horn and the anterior centrum semiovale, and then terminated at the DLPFC. Thalamocortical connections to the VLPFC also ascended through the anterior thalamic radiation via the anterior limb and genu of the upper internal capsule and the corona radiata around the anterior horn, and then terminated at the VLPFC. Thalamocortical connections to the OFC passed horizontally through the anterior thalamic radiation via the anterior limb and genu of the lower internal capsule and terminated at the OFC (Fig. 1). At the centrum semiovale level, thalamocortical connections to the DLPFC were located anterior to the genu of the corpus callosum. By contrast, at the corona radiata level, thalamocortical connections to the VLPFC were located around the frontal horn of the lateral ventricle anterior to the thalamocortical connection to the DLPFC. At the internal capsule, the thalamocortical connections to the OFC were located at the lower internal capsule below the thalamocortical connections to the DLPFC and VLPFC, which were usually located at the upper internal capsule.
Mean values for FA, MD, and tract volume of DLPFC were 0.33±0.03, 0.85±0.06, and 930.03±435.70, respectively, and those of VLPFC were 0.33±0.02, 0.85±0.05, and 1379.81±546.09, respectively. In the OFC, average FA, mean diffusivity, and tract volume were 0.33±0.02, 0.83±0.05, and 2139.00±802.62, respectively. In terms of FA and mean diffusivity, no significant differences were observed between the DLPFC, VLPFC, and OFC (p>0.05) (Table 1). Furthermore, no significant difference was observed between average DLPFC and VLPFC tract volume (p>0.05), but average OFC tract volume was significantly greater than DLPFC and VLPFC tract volume (p<0.05) (Table 1).
In the current study, thalamocortical connections between thalamic MD and DLPFC, VLPFC, and OFC in human brain were reconstructed using DTT. In addition, we characterized thalamocortical neural tracts and their courses between thalamic MD and three PFCs. Thalamocortical connections to DLPFC were located at the centrum semiovale anterior to the genu of the corpus callosum, and thalamocortical connections to VLPFC were located around the frontal horn of the lateral ventricle anterior to the thalamocortical connections to DLPFC at the anterior corona radiata. At the internal capsule level, thalamocortical connections to OFC were located at the lower anterior limb and genu of the internal capsule below the thalamocortical connections to the DLPFC and VLPFC, which were mainly located at the upper anterior limb and genu of the internal capsule. Thus, these thalamocortical connections had different pathways at the centrum semiovale, corona radiata, and internal capsule levels in the order of DLPFC, VLPFC, and OFC, that is, from an upper to a lower level.
Thalamocortical connections between thalamic MD and DLPFC, VLPFC, and OFC have been described in many animal studies, the majority of which focused on connectivity between thalamic nuclei and PFC.11,12,13,14,15,16,17 Some DTI studies on human brain have been reported to date.18,19 Using DTT, Klein, et al.19 found a high probability of connection between parvicellular MD and lateral PFC, between the fibrous part of MD and lateral OFC, and between the caudodorsal part of MD and the medial PFC in human brain. In 2011, Eckert, et al.18 reported a different connectivity of MD and of the centromedian-parafascicular complex of thalamus to PFC and subcortical regions, and that thalamic MD is more frequently connected to PFC than the centromedian-parafascicular complex of thalamus. Recently, using DTI, Kubota, et al.30 reconstructed entire thalamocirtocal pathway, thalamolateral and thalamomedial prefrontal pathway and thalamo-orbitofrontal pathway in schizophrenia patients, and found that schizophrenia patients showed decreased FA value in the thalamo-orbitofronal pathway compared with normal healthy subjects. We performed this study because these previous two DTI studies provided little information on the neural pathways of thalamocortical connections, which are clinically important because they are involved in frontal network syndrome as well as in many psychiatric diseases.1
In conclusion, we identified thalamocortical connections between MD of thalamus and three PFCs in human brain, using DTT. We believe that the methodology used and results of this study would be helpful for researchers in the neuroscience field and also to those involved in neurorehabilitation and psychiatry. In particular, the described courses of the three thalamocortical connections should be useful to clinicians treating frontal network syndrome. However, this study is limited by our inability to reconstruct thalamocortical connections to the medial PFC, and we consider that this topic is worthy of future study. In addition, further studies on clinical correlations and on the reliabilities and validities of the three thalamocortical connections to PFCs are needed.
Figures and Tables
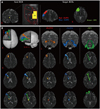 | Fig. 1(A) Seed regions of interest (ROI) were placed on the mediodorsal nucleus (MD) of the thalamus (yellow color). Target ROIs were placed on the dorsolateral prefrontal cortex (DLPFC, red), the ventrolateral prefrontal cortex (VLPFC, blue), and orbitoprefrontal cortex (OFC, green), respectively. (B) The neural pathways of thalamocortical connections between thalamic MD and three prefrontal cortexes are shown at each brain level in a normal subject (a 50-year-old female). |
Table 1
Differences between the DTI Parameters of Thalamocortical Connections between the Medial Nucleus of the Thalamus and the Prefrontal Cortex

FA, fractional anisotropy; DLPFC, dorsolateral prefrontal cortex; VLPFC, ventrolateral prefrontal cortex; OFC, orbitofrontal cortex; DTI, diffusion tensor imaging.
DTI parameters are presented as means (standard deviations). One way analysis of variance (ANOVA) was used to compare DTI parameters.
*In the DLPFC and VLPFC.
†In the DLFPC and OFC.
‡In the VLPFC and OFC.
§p<0.05.
References
1. Mesulam MM. Principles of behavioral and cognitive neurology. 2nd ed. New York: Oxford University;2000.
2. Clark DL, Boutros NN, Mendez MF. The brain and behavior: an introduction to behavioral neuroanatomy. 3rd ed. New York: Cambridge University;2010.
3. Sakagami M, Pan X. Functional role of the ventrolateral prefrontal cortex in decision making. Curr Opin Neurobiol. 2007; 17:228–233.

4. Davidson RJ, Putnam KM, Larson CL. Dysfunction in the neural circuitry of emotion regulation--a possible prelude to violence. Science. 2000; 289:591–594.

6. Fuster JM. The Prefrontal Cortex. 4th ed. New York: Academic Press;2008.
7. De Witte L, Brouns R, Kavadias D, Engelborghs S, De Deyn PP, Mariën P. Cognitive, affective and behavioural disturbances following vascular thalamic lesions: a review. Cortex. 2011; 47:273–319.

8. Liebermann D, Ploner CJ, Kraft A, Kopp UA, Ostendorf F. A dysexecutive syndrome of the medial thalamus. Cortex. 2013; 49:40–49.

9. Summers MJ. Neuropsychological consequences of right thalamic haemorrhage: case study and review. Brain Cogn. 2002; 50:129–138.

10. Rose SE, Chalk JB, Janke AL, Strudwick MW, Windus LC, Hannah DE, et al. Evidence of altered prefrontal-thalamic circuitry in schizophrenia: an optimized diffusion MRI study. Neuroimage. 2006; 32:16–22.

11. Ray JP, Price JL. The organization of projections from the mediodorsal nucleus of the thalamus to orbital and medial prefrontal cortex in macaque monkeys. J Comp Neurol. 1993; 337:1–31.

12. Barbas H, Henion TH, Dermon CR. Diverse thalamic projections to the prefrontal cortex in the rhesus monkey. J Comp Neurol. 1991; 313:65–94.

13. Goldman-Rakic PS, Porrino LJ. The primate mediodorsal (MD) nucleus and its projection to the frontal lobe. J Comp Neurol. 1985; 242:535–560.

14. Giguere M, Goldman-Rakic PS. Mediodorsal nucleus: areal, laminar, and tangential distribution of afferents and efferents in the frontal lobe of rhesus monkeys. J Comp Neurol. 1988; 277:195–213.

15. Reep RL, Corwin JV, King V. Neuronal connections of orbital cortex in rats: topography of cortical and thalamic afferents. Exp Brain Res. 1996; 111:215–232.

16. Ongür D, Price JL. The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys and humans. Cereb Cortex. 2000; 10:206–219.

17. Contini M, Baccarini M, Borra E, Gerbella M, Rozzi S, Luppino G. Thalamic projections to the macaque caudal ventrolateral prefrontal areas 45A and 45B. Eur J Neurosci. 2010; 32:1337–1353.

18. Eckert U, Metzger CD, Buchmann JE, Kaufmann J, Osoba A, Li M, et al. Preferential networks of the mediodorsal nucleus and centromedian-parafascicular complex of the thalamus--a DTI tractography study. Hum Brain Mapp. 2012; 33:2627–2637.

19. Klein JC, Rushworth MF, Behrens TE, Mackay CE, de Crespigny AJ, D'Arceuil H, et al. Topography of connections between human prefrontal cortex and mediodorsal thalamus studied with diffusion tractography. Neuroimage. 2010; 51:555–564.

20. Mamah D, Conturo TE, Harms MP, Akbudak E, Wang L, McMichael AR, et al. Anterior thalamic radiation integrity in schizophrenia: a diffusion-tensor imaging study. Psychiatry Res. 2010; 183:144–150.

21. Wakana S, Jiang H, Nagae-Poetscher LM, van Zijl PC, Mori S. Fiber tract-based atlas of human white matter anatomy. Radiology. 2004; 230:77–87.

22. Nagae LM, Hoon AH Jr, Stashinko E, Lin D, Zhang W, Levey E, et al. Diffusion tensor imaging in children with periventricular leukomalacia: variability of injuries to white matter tracts. AJNR Am J Neuroradiol. 2007; 28:1213–1222.

23. Yin H, Cheng SH, Zhang J, Ma L, Gao Y, Li D, et al. Corticospinal tract degeneration in amyotrophic lateral sclerosis: a diffusion tensor imaging and fibre tractography study. Ann Acad Med Singapore. 2008; 37:411–415.
24. Kwon HG, Hong JH, Hong CP, Lee DH, Ahn SH, Jang SH. Dentatorubrothalamic tract in human brain: diffusion tensor tractography study. Neuroradiology. 2011; 53:787–791.

25. Kwon HG, Hong JH, Jang SH. Mammillothalamic tract in human brain: diffusion tensor tractography study. Neurosci Lett. 2010; 481:51–53.

26. Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004; 23:Suppl 1. S208–S219.

27. Johansen-Berg H, Behrens TE, Sillery E, Ciccarelli O, Thompson AJ, Smith SM, et al. Functional-anatomical validation and individual variation of diffusion tractography-based segmentation of the human thalamus. Cereb Cortex. 2005; 15:31–39.

28. Petrides M. Lateral prefrontal cortex: architectonic and functional organization. Philos Trans R Soc Lond B Biol Sci. 2005; 360:781–795.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download