Abstract
Purpose
The underlying cause of myasthenia gravis (MG) is unknown, although it likely involves a genetic component. However, no common genetic variants have been unequivocally linked to autoimmune MG. We sought to identify the genetic variants associated with an increased or decreased risk of developing MG in samples from a Korean Multicenter MG Cohort.
Materials and Methods
To determine new genetic targets related to autoimmune MG, a whole genome-based single nucleotide polymorphisms (SNP) analysis was conducted using an Axiom™ Genome-Wide ASI 1 Array, comprising 598375 SNPs and samples from 109 MG patients and 150 neurologically normal controls.
Results
In total, 641 SNPs from five case-control associations showed p-values of less than 10-5. From regional analysis, we selected seven candidate genes (RYR3, CACNA1S, SLAMF1, SOX5, FHOD3, GABRB1, and SACS) for further analysis.
Conclusion
The present study suggests that a few genetic polymorphisms, such as in RYR3, CACNA1S, and SLAMF1, might be related to autoimmune MG. Our findings also encourage further studies, particularly confirmatory studies with larger samples, to validate and analyze the association between these SNPs and autoimmune MG.
Go to : 
Myasthenia gravis (MG) is a defect of nerve-muscle transmission caused by autoantibodies to different components of the postsynaptic membrane.1 Similar to other autoimmune diseases, tolerance breakdown is a highly complex and multifactorial process, resulting from the alteration of multiple pathways. As previously demonstrated in other autoimmune diseases,2 genetic factors are thought to play an important role in the pathogenesis of MG, despite its limited heritability. MG patients, as well as their close relatives, are often affected by other autoimmune diseases,3 which strongly suggests shared genetic mechanisms.
Among the genetic factors investigated in MG, major histocompatibility complex holds a unique place. It has been reproducibly associated with MG in many different populations and it shows large effects. However, which genes are responsible for the association of this large and essential region is not yet known. Various genes associated with MG have been identified, including cholinergic receptor nicotinic alpha 1 (CHRNA1),4,5 protein tyrosine phosphatase, non-receptor type 22 (PTPN22),6,7 and cytotoxic T-lymphocyte-associated protein 4 (CTLA4).8,9 Despite demonstrating associations among many genes and MG,10 their roles in MG remain elusive and precise identification thereof at the molecular level remains challenging. Identification of specific genetic variants associated with autoimmune MG will improve our understanding of fundamental disease mechanisms.
To identify genetic factors that might be relevant in the pathogenesis of autoimmune MG, we genotyped 598375 unique single nucleotide polymorphisms (SNPs) across the genome using an Axiom™ Genome-Wide ASI 1 Array Chip (Affymetrix, Santa Clara, CA, USA) in 109 patients with autoimmune MG and 150 neurologically normal controls.
Go to : 
Samples were obtained from four university hospitals (Konyang University Hospital, Gangnam Severance Hospital, Chugnam National University Hospital, and Pusan National University Hospital). All were mutually unrelated Korean subjects and provided written informed consent to participate in the study, as stipulated by the respective Institutional Review Boards. Additionally, the study was performed according to the Declaration of Helsinki.
DNA from 109 unique and mutually unrelated Korean individuals diagnosed with autoimmune MG was selected for the analysis. Their diagnoses were based on clinical signs of fluctuating weakness of voluntary muscles with fatigability and either a positive response to neostigmine or electrophysiological evidence of a greater than 10% decrease in the negative-peak amplitude of compound muscle action potential upon supramaximal repetitive (2 or 3 Hz) nerve stimulation. A positive response to the neostigmine test was defined as follows: muscle weakness caused by MG is temporarily reversed following the administration of neostigmine.
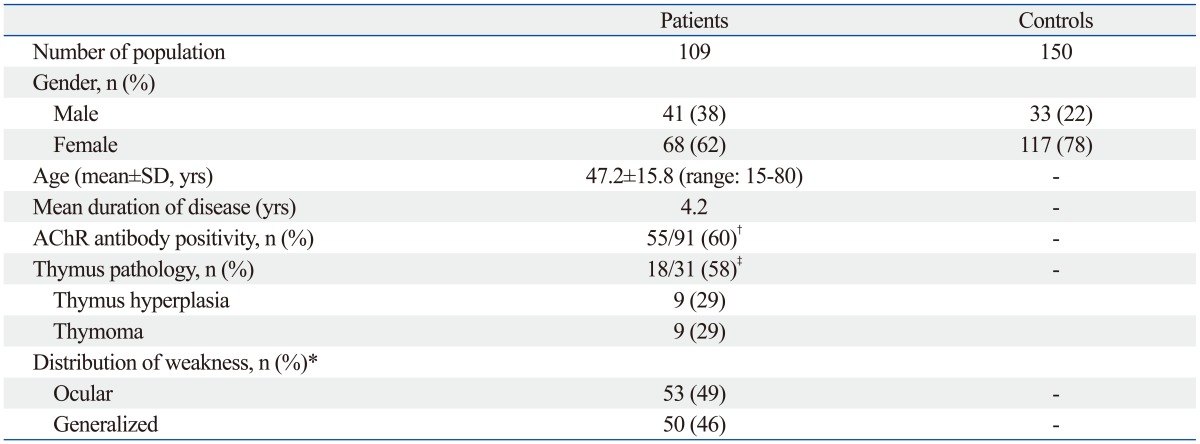
Eligible patients were classified as having ocular MG or generalized MG according to the clinical distribution of muscle involvement. Patients were classified as having a generalized disease if there was any weakness on exertion of the facial (except the orbicularis oculi), oropharyngeal, neck, respiratory, axial, or limb muscles, which could be identified either subjectively or during neurological examination. Ocular MG was defined as follows: ocular disturbances are the first and sole manifestation of MG and no progression to generalized MG within the first 3 years has been recorded. Acetylcholine receptor (AChR) binding antibodies determined by radioimmunoassay were considered positive at ≥0.5 nmol/L and AChR blocking antibodies determined by radioreceptor assay were considered positive ≥31% blocking. We excluded MG patients with autoimmune diseases other than MG.
The genotype data of normal controls were obtained from the samples of another study, which involved mutually unrelated Korean individuals within the Republic of Korea. All participants underwent a detailed medical history interview. None had history of MG, amyotrophic lateral sclerosis (ALS), Alzheimer's disease, ataxia, autism, bipolar disorder, dementia, dystonia, or Parkinson's disease. All participants were interviewed for family history in detail. None had any first-degree relative with a known primary neurological disorder, including MG, ALS, ataxia, autism, dystonia, Parkinson's disease, or schizophrenia. The control and the case cohorts were drawn from the same ethnic origin and were not matched by age or sex with the MG samples. The control cohort consisted of 33 men and 117 women.
The ages of the patients at onset of the disease ranged from 12 to 62 years of age (mean=43.6 years). The disease onset was defined as the time when weakness was first noted. The MG patients were classified by disease type (ocular or generalized), the presence or absence of thymic hyperplasia/thymoma as determined by a thymic pathology study, and whether anti-AChR blocking or binding antibody was positive or negative.
After obtaining consent, each participant provided a venous blood sample that was collected in ethylene diamine tetraacetic acid-coated tubes. All samples were labeled with an anonymous code number linked only to demographic and clinical information.
The patients' genomic DNA was extracted from peripheral blood using a standard protocol. We genotyped 598375 SNPs from 109 MG patients using an Axiom™ Genome-Wide ASI 1 Array Chip (Affymetrix, Santa Clara, CA, USA), according to the manufacturer's instructions. Data from 150 control samples were obtained from another study and were assayed with an Axiom™ Genome-Wide ASI 1 Array Chip. The Axiom ASI Chip provides 88% common variant coverage in Asians and can provide 74% rare variant [minor allele frequency (MAF) ≤5%] coverage in Asians.
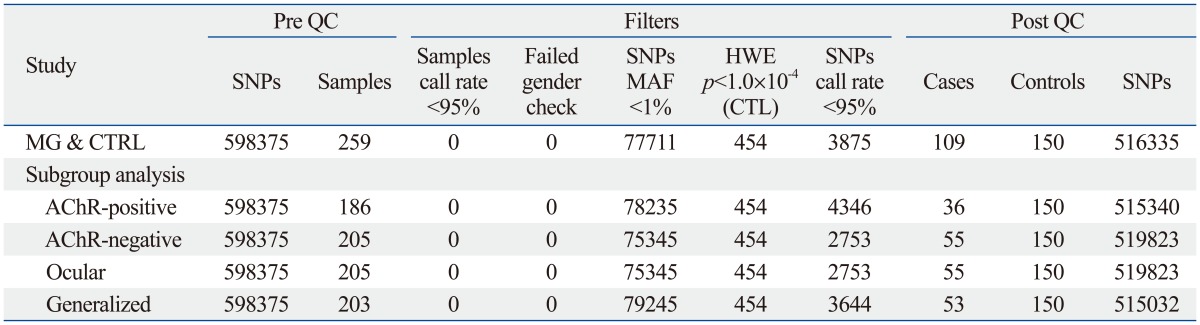
To reduce potential concerns for batch effects and the possibility of false associations, we applied highly stringent quality control measures to select SNPs for use in the case-control datasets. The quality control procedures were performed on each of the 598K SNPs before the association tests were conducted. The SNP set was filtered based on the genotype call rates (≥90%) and MAF (≥0.01). Hardy-Weinberg equilibrium (HWE) was calculated for individual SNPs using an exact test. All of the SNPs reported in this manuscript demonstrated HWE p values >10-4. After filtering, 502500 polymorphic SNPs were analyzed on chromosome 1 through chromosome 22 and 13850 and 13835 SNPs for chromosome X and Y, respectively. The average call rate for the filtered SNPs was 99.3%. We removed the patients if their percentage of missing genotypes was more than 5% or if there was evidence of possible contamination in their DNA samples.
We evaluated associations between SNP markers and autoimmune MG. First, we performed the association tests on all MG patients and control subjects, as well as undertook further analysis of samples from subgroups such as AChR-positive, AChR-negative, ocular MG, and generalized MG patients. As the sample size was relatively small in our study, multiple hit SNPs from all association tests had more power than a single association test. For each SNP, we computed a series of summary statistics and association tests using the PLINK toolset. Five association tests were computed: a two degrees of freedom genotypic test in 2×3 tables, a dominant model, an additive model (Cochran-Armitage trend test), a recessive model, and the basic allelic test. Odds ratios, as well as upper and lower bound 95% CIs, were computed for each SNP's minor allele.
Go to : 
In total, 109 autoimmune MG patients with complete genotypic and phenotypic data were enrolled in this study. All were used for the subsequent association analyses. The baseline characteristics of the 109 patients are summarized in Table 1. At the time of diagnosis, their ages in years were 47.2±15.8 and their mean disease duration was 4.2 years. Overall, 68 patients (62%) were women. Among 109 cases, 50 (46%) presented with generalized symptoms, 53 (49%) had a purely ocular manifestation, and the remaining six could not be classified due to uncertain clinical information. Of the 91cases in which the AChR antibody test was performed, 55 cases (60%) were positive. Of the 31 cases in which the thymic pathology study was performed, 18 cases (58%) had thymic hyperplasia/thymoma. Genome-wide association (GWA) analyses were performed after the quality control (QC) procedure to test the association with MG. In the sample QC, all 109 samples from the MG cohort passed the genotype quality threshold and were included in the final analysis.
The quality control criteria for SNP selection were 1) minor allele frequency >0.01, 2) p>10-4 in the Hardy-Weinberg Equilibrium test, and 3) Cluster QC >10-4. Of the 598375 SNPs studied, 516335 SNPs passed the QC (representing 86.3% of all SNPs assayed) in the MG and control association test. A quantile-quantile plot for the genome-wide phase, in which the observed -log10
p-values were plotted against the expectation under the null hypothesis, showed an excess of significant associations. The QC for subgroup analysis also was performed in this manner. Those passing the QC test included 515340 SNPs (86.1%) in the AChR-positive MG and control association test, 519823 SNPs (86.9%) in the AChR antibody-negative MG and control association test, 515032 SNPs (86.1%) in the ocular MG and control association test, and 515615 SNPs (86.2%) in the generalized MG and control association test. A summary of the quality control statistics of genome-wide data is presented in Table 2.
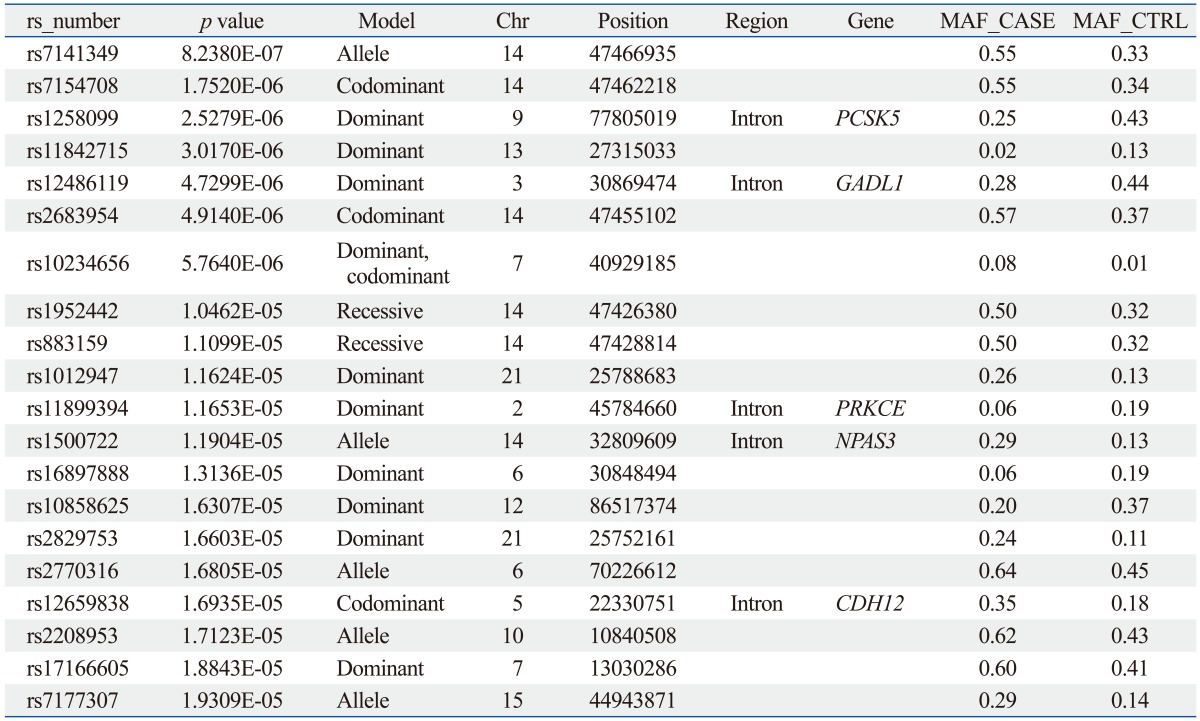
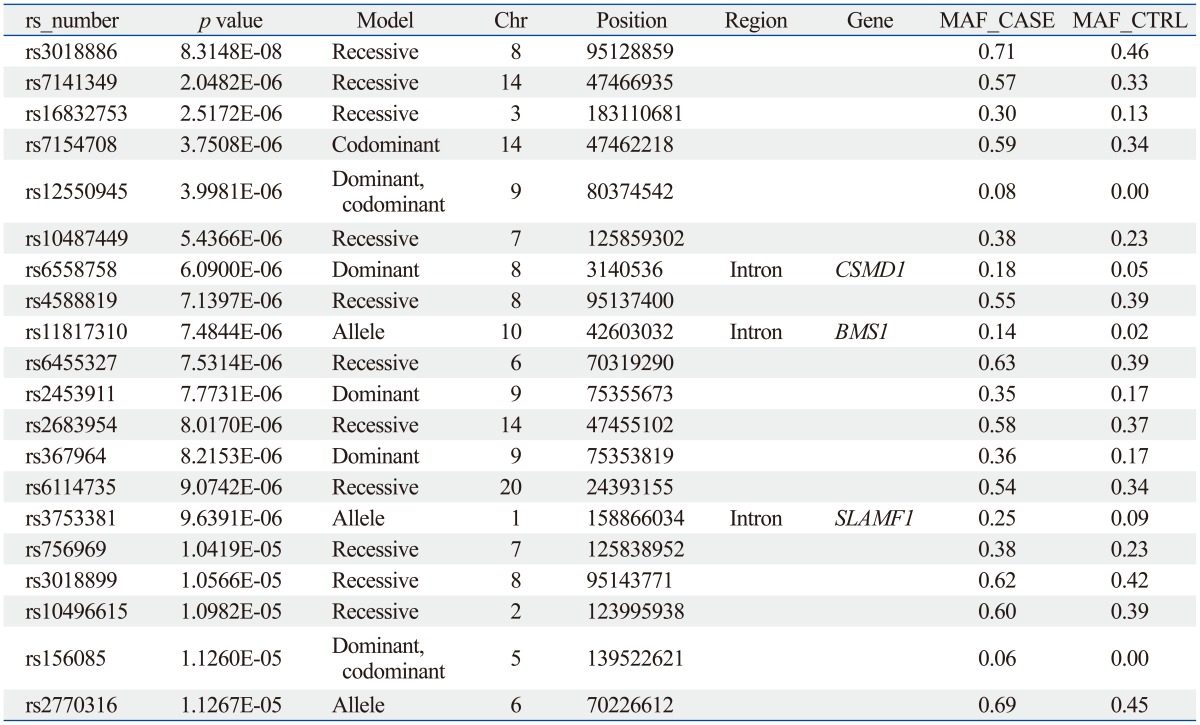
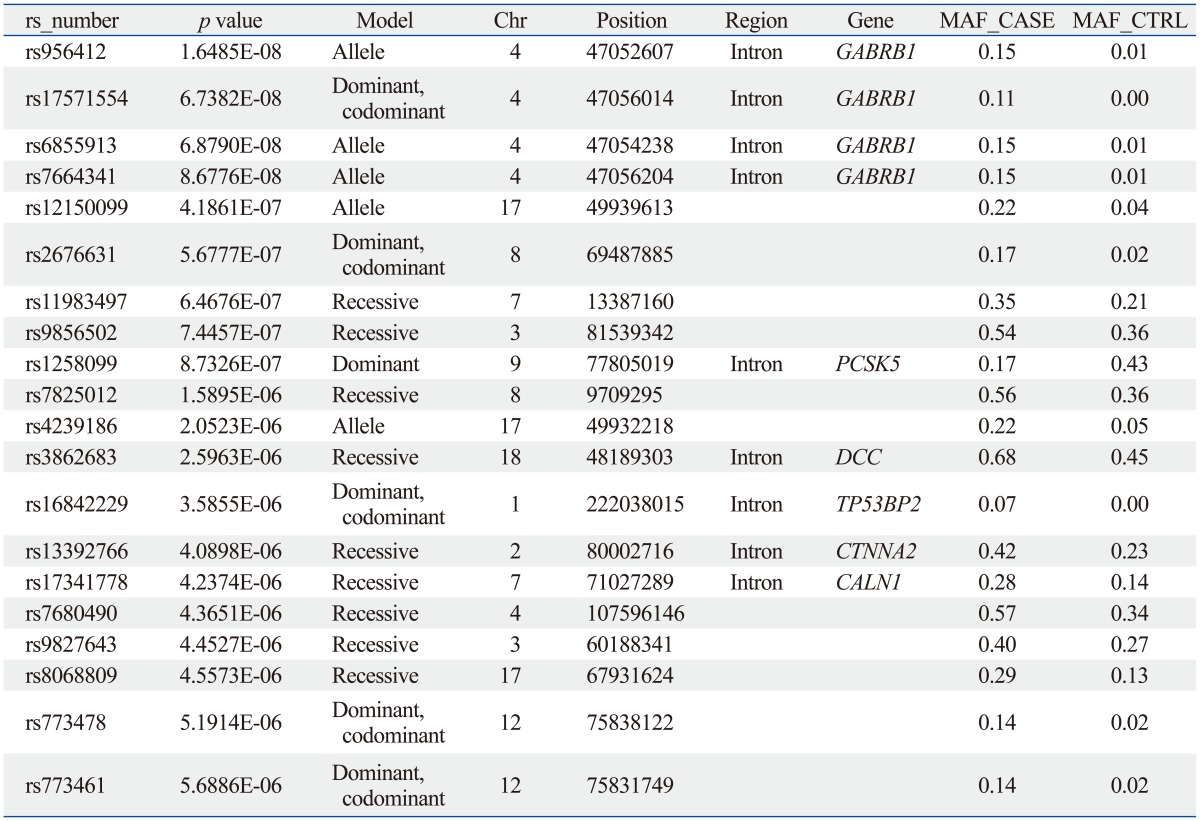
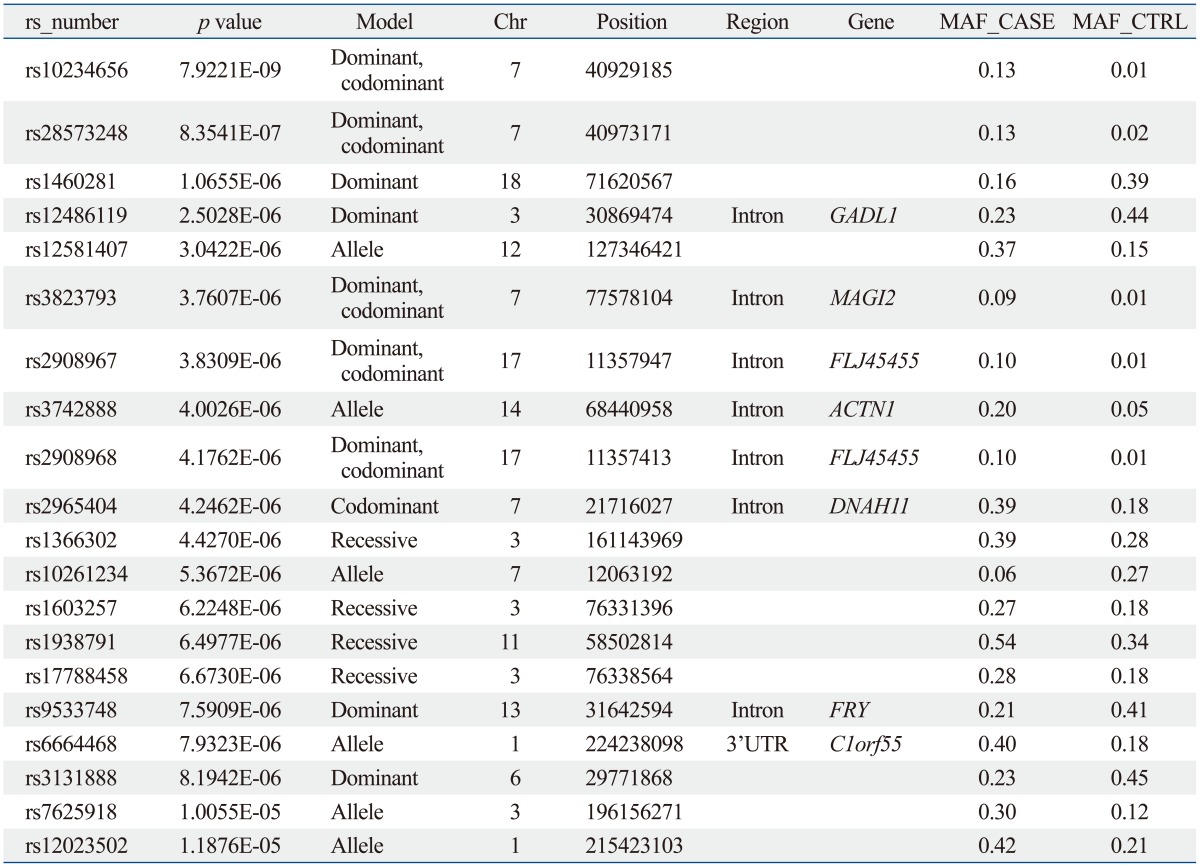
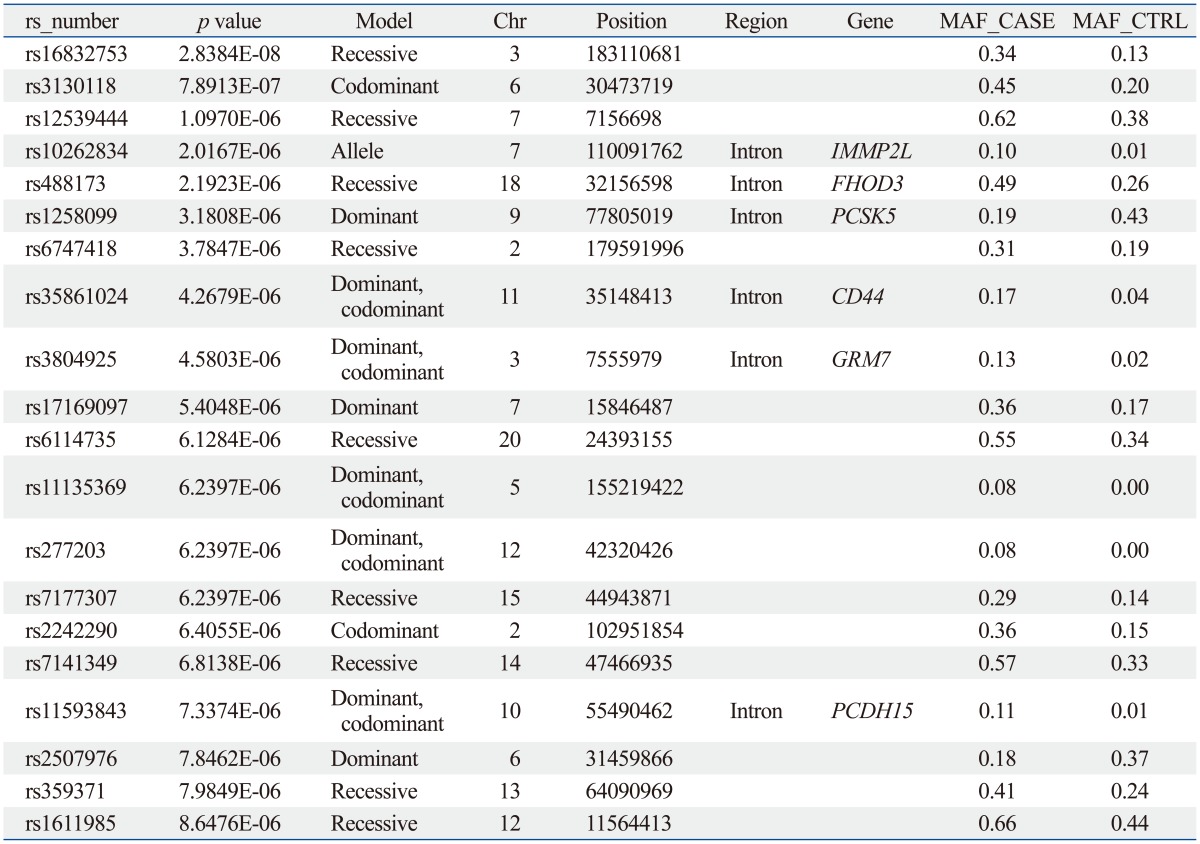
The sample size of the total MG data set was too small to select a candidate SNP for MG. Thus, we repeated the statistical analysis for four subgroups of patients with generalized MG (n=50), ocular MG (n=53), AChR antibody positive MG (n=55), and AChR antibody negative MG (n=36). In every case, the subgroup was compared with the full control cohort (n=150). Of these, 641 SNPs showed significant association in all case-control association tests based on the pre-set level of significance (p-values less than 10-5); the top 20 p-values of SNPs are shown in Table 3,4,5,6,7.
From these results, we selected candidate association regions in two different ways. First, we selected common hit regions from all association datasets. Second, we performed a regional analysis on each case-control association dataset, as significant peaks usually tend to occur near each other. Finally, we selected seven genes (RYR3, CACNA1S, SLAMF1, SOX5, FHOD3, GABRB1, and SACS) for further analysis.
The SLAMF1, CACNA1S, and RYR3 genes were selected from the analysis between the AChR antibody positive MG cases and controls. The GABRB1 and SACS genes were selected from the analysis between AChR antibody negative MG cases and controls. The SOX and FHOD3 genes were selected from the analysis between generalized MG cases and controls.
Go to : 
In this study, we generated genome-wide SNP data consisting of more than 500K genotypes from multicenter cohort patients with autoimmune MG and controls derived from the samples used in another study. The use of tagging SNPs greatly improves the power of association studies, as the same information from a larger number of SNPs can be gathered by genotyping only one subset of loci. The Axiom ASI Array provides 88% common variant coverage in Asians. Furthermore, it can provide 74% rare variant (MAF ≤5%) coverage in Asians. Tagged SNPs on the Axiom Genome-Wide ASI Array plate were selected from -515K HapMap markers, -72K 1000 Genome project markers, and -10K other source markers, including -2K indels. Thus, most known genetic variations identified by the HapMap consortium were assessed in this project. Furthermore, the SNPs on the Axiom ASI SNP Chip comprise a large representation of functionally relevant markers, making the assay ideally suited for assessing whole-genome associations.
Our data provided a power of 80% to detect an allelic association with an odds ratio of more than 2.75 and less than 0.33 at an asymptotic p-value of 0.000001. This calculation was based on the average observed MAF of 23.7% and assumed either that the causal variant is typed or that there is complete and efficient tagging of common variation by the genotyped SNPs. The limited power of our study was a consequence of the small sample size, though the digital nature of the data allows it to be supplemented with genotype information from additional sample series, thereby increasing the power over time.
Analysis of our data revealed 641 SNPs with a p-value less than 0.00001 and with odds ratios ranging from 0.5 (95% CI 0.4-0.7) to 2.1 (95% CI 1.5-3.0). A Bonferroni correction based on the 502500 independent tests used for analysis indicated that a pre-correction p-value of less than 9.9×10-8 would be needed to provide a corrected significant p-value of less than 0.05. Based on this threshold, only one SNP (rs10234656, p-value=7.9×10-9 in the subgroup analysis for ocular MG) was significant after the correction. However, this SNP showed the highest p-value within only a single region, and we did not focus on further analysis because significant peaks usually tend to occur near each other in genome-wide association study (GWAS) data. Excluding this one SNP, none of the other SNPs listed were significant after the correction. This suggests that the MG phenotype is not driven by a single powerful locus.
However, other possible explanations exist as to why our study might not have detected an SNP that convincingly relates to disease risk. Firstly, the Bonferroni correction is known to be too conservative, especially in whole-genome association studies where dependence (i.e., linkage disequilibrium) among SNPs is ignored.11 Second, the power of our study was limited by its sample size and the lack of age-matched and sex-matched controls. Finally, it is possible that autoimmune MG consists of a diverse group of clinically indistinguishable disorders, each driven by different loci. Such disease and locus heterogeneity would interfere with the ability of whole-genome association studies to detect the SNPs associated with increased risk of disease. Therefore, we conducted a regional analysis on each case-control association dataset and finally selected seven genes (RYR3, CACNA1S, SLAMF1, SOX5, FHOD3, GABRB1, and SACS) for further analysis.
MG is an autoimmune disease with impaired neuromuscular transmission mainly due to the effect of autoantibodies on the AChR.12 Recently, dysregulation of sarcoplasmic reticulum Ca2+ release has been associated with muscle fatigue.13 An electrophysiological study showed that fatigue in MG patients was caused not only by abnormal neuromuscular transmission, but also by impairment of excitation-contraction (E-C) coupling.14 In the process of E-C coupling in skeletal muscles, the ryanodine receptor (RyR) and the dihydropyridine receptor (DHPR/CACNA1S) function as Ca2+ channels.15
SLAMF1 is an important genetic susceptibility locus for autoantibody production.16
SLAMF1 leads to IFN-γ production of CD4+ T cells,17 which is associated with pathogenesis and immunoregulation of MG. However, speculating on the plausibility and the biological significance of these candidate loci is premature, as additional genetic data is needed to confirm the notion that the E-C coupling gene or SLAMF1 gene are associated with autoimmune MG. Furthermore, as we have learned in the study of Alzheimer's, the identification of even a strong risk factor gene does not immediately provide a cell biological explanation for disease pathogenesis.
The first GWAS data for MG, published earlier this year,18 showed that HLA-B*08, PTPN22, and TNIP1 are associated with early onset MG (EOMG). None of the 641 significantly associated SNPs from all the association data sets were located in any of the known autoimmune MG genes or loci in our study. The discrepancy may be caused by phenotypic differences between the two studies. They only enrolled EOMG patients in their study, and we included all MG patients regardless of age at onset. In addition, ethnic differences could also result in varying association results. These probabilities indicated that the genetic factors contributing to the development or pathogenesis of MG are very complicated.
The ability of whole-genome association studies to identify the genetic variants underlying a disease is well recognized. For example, the analysis of 100000 SNPs in only 96 cases recently established that the variants in the complement factor H gene account for a significant proportion of age-related macular degeneration.19 Despite this finding, data generated in whole-genome association studies should be interpreted cautiously because of the inevitably high false-positive rate that occurs whenever several hundred thousand tests are undertaken on the same dataset. For example, Maraganore, et al.20 identified 13 putative SNPs that seemed to be associated with Parkinson's disease, but none have been confirmed in subsequent independent studies.21,22,23,24,25
To address the concern regarding false positive associations, it is our intention to replicate our current study using the same Axiom Genome-Wide ASI 1 Array in an additional sample set of equal or greater size drawn from the Korean population. Joint analysis of these data sets will significantly increase our power to detect an allelic association, and loci that are common to both Korean and other Asian populations will be further analyzed to determine the precise genetic variation that conveys increased susceptibility to autoimmune MG.
In summary, we report data from a whole-genome association study of a large group of autoimmune MG patients and neurologically normal control samples. In total, 641 loci showing potentially significant associations with MG were identified with a genotypic p-value of less than 0.00001. To overcome the possibility of false-positive findings, this study will be replicated in a separate cohort of 120 Korean MG cases-control pairs. From regional analysis, we selected seven genes (RYR3, CACNA1S, SLAMF1, SOX5, FHOD3, GABRB1, and SACS) for further analyses. Among the seven genes, we suggest that three genes (RYR3, CACNA1S, and SLAMF1) are associated with autoimmune MG. However, speculating on the plausibility and the biological significance of these candidate loci is premature, as additional genetic data are needed to confirm the notion that the E-C coupling gene or SLAMF1 gene are associated with autoimmune MG.
Go to : 
ACKNOWLEDGEMENTS
This study was supported by a faculty research grant of Yonsei University College of Medicine for 2011 (6-2011-0075).
Go to : 
References
1. Meriggioli MN, Sanders DB. Autoimmune myasthenia gravis: emerging clinical and biological heterogeneity. Lancet Neurol. 2009; 8:475–490. PMID: 19375665.

2. Gregersen PK, Behrens TW. Genetics of autoimmune diseases--disorders of immune homeostasis. Nat Rev Genet. 2006; 7:917–928. PMID: 17139323.
3. Palmisani MT, Evoli A, Batocchi AP, Bartoccioni E, Tonali P. Myasthenia gravis and associated autoimmune diseases. Muscle Nerve. 1994; 17:1234–1235. PMID: 7935540.
4. Garchon HJ, Djabiri F, Viard JP, Gajdos P, Bach JF. Involvement of human muscle acetylcholine receptor alpha-subunit gene (CHRNA) in susceptibility to myasthenia gravis. Proc Natl Acad Sci U S A. 1994; 91:4668–4672. PMID: 7910962.

5. Giraud M, Taubert R, Vandiedonck C, Ke X, Lévi-Strauss M, Pagani F, et al. An IRF8-binding promoter variant and AIRE control CHRNA1 promiscuous expression in thymus. Nature. 2007; 448:934–937. PMID: 17687331.

6. Vandiedonck C, Capdevielle C, Giraud M, Krumeich S, Jais JP, Eymard B, et al. Association of the PTPN22*R620W polymorphism with autoimmune myasthenia gravis. Ann Neurol. 2006; 59:404–407. PMID: 16437561.
7. Greve B, Hoffmann P, Illes Z, Rozsa C, Berger K, Weissert R, et al. The autoimmunity-related polymorphism PTPN22 1858C/T is associated with anti-titin antibody-positive myasthenia gravis. Hum Immunol. 2009; 70:540–542. PMID: 19406179.

8. Chuang WY, Ströbel P, Gold R, Nix W, Schalke B, Kiefer R, et al. A CTLA4high genotype is associated with myasthenia gravis in thymoma patients. Ann Neurol. 2005; 58:644–648. PMID: 16178018.

9. Wang XB, Kakoulidou M, Qiu Q, Giscombe R, Huang D, Pirskanen R, et al. CDS1 and promoter single nucleotide polymorphisms of the CTLA-4 gene in human myasthenia gravis. Genes Immun. 2002; 3:46–49. PMID: 11857062.

10. Giraud M, Vandiedonck C, Garchon HJ. Genetic factors in autoimmune myasthenia gravis. Ann N Y Acad Sci. 2008; 1132:180–192. PMID: 18567868.

11. Van Steen K, McQueen MB, Herbert A, Raby B, Lyon H, Demeo DL, et al. Genomic screening and replication using the same data set in family-based association testing. Nat Genet. 2005; 37:683–691. PMID: 15937480.

12. Conti-Fine BM, Milani M, Kaminski HJ. Myasthenia gravis: past, present, and future. J Clin Invest. 2006; 116:2843–2854. PMID: 17080188.

13. Bellinger AM, Mongillo M, Marks AR. Stressed out: the skeletal muscle ryanodine receptor as a target of stress. J Clin Invest. 2008; 118:445–453. PMID: 18246195.

14. Pagala M, Nandakumar NV, Venkatachari SA, Ravindran K, Amaladevi B, Namba T, et al. Mechanisms of fatigue in normal intercostal muscle and muscle from patients with myasthenia gravis. Muscle Nerve. 1993; 16:911–921. PMID: 8355722.

15. Takeshima H, Iino M, Takekura H, Nishi M, Kuno J, Minowa O, et al. Excitation-contraction uncoupling and muscular degeneration in mice lacking functional skeletal muscle ryanodine-receptor gene. Nature. 1994; 369:556–559. PMID: 7515481.

16. Chan AY, Westcott JM, Mooney JM, Wakeland EK, Schatzle JD. The role of SAP and the SLAM family in autoimmunity. Curr Opin Immunol. 2006; 18:656–664. PMID: 17011767.

17. Cocks BG, Chang CC, Carballido JM, Yssel H, de Vries JE, Aversa G. A novel receptor involved in T-cell activation. Nature. 1995; 376:260–263. PMID: 7617038.

18. Gregersen PK, Kosoy R, Lee AT, Lamb J, Sussman J, McKee D, et al. Risk for myasthenia gravis maps to a (151) Pro→Ala change in TNIP1 and to human leukocyte antigen-B*08. Ann Neurol. 2012; 72:927–935. PMID: 23055271.
19. Klein RJ, Zeiss C, Chew EY, Tsai JY, Sackler RS, Haynes C, et al. Complement factor H polymorphism in age-related macular degeneration. Science. 2005; 308:385–389. PMID: 15761122.

20. Maraganore DM, de Andrade M, Lesnick TG, Strain KJ, Farrer MJ, Rocca WA, et al. High-resolution whole-genome association study of Parkinson disease. Am J Hum Genet. 2005; 77:685–693. PMID: 16252231.

21. Fung HC, Scholz S, Matarin M, Simón-Sánchez J, Hernandez D, Britton A, et al. Genome-wide genotyping in Parkinson's disease and neurologically normal controls: first stage analysis and public release of data. Lancet Neurol. 2006; 5:911–916. PMID: 17052657.

22. Elbaz A, Nelson LM, Payami H, Ioannidis JP, Fiske BK, Annesi G, et al. Lack of replication of thirteen single-nucleotide polymorphisms implicated in Parkinson's disease: a large-scale international study. Lancet Neurol. 2006; 5:917–923. PMID: 17052658.

23. Farrer MJ, Haugarvoll K, Ross OA, Stone JT, Milkovic NM, Cobb SA, et al. Genomewide association, Parkinson disease, and PARK10. Am J Hum Genet. 2006; 78:1084–1088. PMID: 16685661.

24. Goris A, Williams-Gray CH, Foltynie T, Compston DA, Barker RA, Sawcer SJ. No evidence for association with Parkinson disease for 13 single-nucleotide polymorphisms identified by whole-genome association screening. Am J Hum Genet. 2006; 78:1088–1090. PMID: 16685662.

25. Li Y, Rowland C, Schrodi S, Laird W, Tacey K, Ross D, et al. A case-control association study of the 12 single-nucleotide polymorphisms implicated in Parkinson disease by a recent genome scan. Am J Hum Genet. 2006; 78:1090–1092. PMID: 16685663.

Go to : 




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download