Abstract
Purpose
Endoscopic papillectomy (EP) is currently employed for the treatment of ampullary adenoma. This study aimed to evaluate the clinical, endoscopic, and histologic characteristics related to complications and long-term outcomes of EP.
Materials and Methods
Thirty-nine patients underwent EP for ampullary adenoma. Patients were grouped according to the occurrence of procedure-related complications: no complication group (n=28) and complication group (n=11).
Results
The overall complication rate was 28.2%. The most common complication was EP-related pancreatitis (n=7). Amylase (p=0.006) and lipase levels (p=0.007), 24 hours after EP, were significantly higher in the complication group, however, these levels did not differ at earlier times. As the tumor progressed from adenoma to cancer, the complete resection was significantly lessened (p=0.032). The duration of antiprotease injection during the hospital stay was significantly longer (p=0.017) and the transfusion requirements were significantly higher (p=0.018) in the complication group. During a median follow-up of 15 months, three lesions (10.3%) recurred among patients with complete resection (n=29) and five lesions (12.8%) recurred among enrolled patients. One patient with progressive recurrence from low-grade dysplasia to adenocarcinoma was noted during a follow-up of 22 months.
Conclusion
If symptoms are present, amylase and lipase levels, 24 hours after EP, could help predict possible EP-related pancreatitis. Histologic diagnosis through resected specimens may result in complete resection. Patients with complications need a longer duration of antiprotease injection during their hospital stay and more transfusions. The recurrence rate was not significantly high in completely resected cases, however, there was a possibility of progressive recurrence.
Ampullary adenomas are rare with a prevalence of 0.04% to 0.12% in autopsy studies,1 and may occur either sporadically or in patients with familiar adenomatous polyposis.2 Ampullary adenomas should be removed completely, because they can potentially undergo an adenoma-carcinoma sequence with a transformation rate to carcinoma of up to 30%;3,4 biopsy of ampullary tumors using endoscopic forceps revealed an alarming 30% false-negative rate for detecting carcinoma in situ and invasive carcinoma.5
Surgical resection, including pancreatoduodenectomy and transduodenal resection, has been considered the standard treatment for ampullary adenomas.6 Recently, endoscopic papillectomy (EP) was employed for the treatment of ampullary adenomas as a feasible alternative procedure, because of considerable perioperative morbidity and mortality of surgical resection.6,7 The main concerns about EP are procedure-related complications and long-term treatment outcomes. The overall complication rate related to EP in previous studies ranged from 8% to 32%,8,9 and the recurrence rate was reported to be 2.8% to 15% during median follow-ups of 14 to 43 months in earlier studies.7,10 Despite relatively high complication and recurrence rates, studies of the characteristics of complications and long-term outcomes of EP are limited due to the rarity of the disease.
This study aimed to evaluate the clinical, endoscopic, and histologic characteristics of complications after EP and long-term treatment outcomes of EP.
From September 2006 to April 2012, 39 consecutive patients with ampullary adenoma underwent EP at a high-volume tertiary referral center. During the same period, 3332 endoscopic retrograde cholangiopancreatography (ERCP) procedures, including 2437 diagnostic and 895 therapeutic procedures, were performed. Thus, 1.2% of all ERCPs and 4.4% of therapeutic ERCPs were carried out for EP. Included patients were grouped according to the occurrence of procedure-related complications: no complication group (without complication, n=28) and complication group (with complication, n=11).
We retrospectively analyzed the clinical, laboratory, endoscopic, and histologic data which were prospectively recorded. Treatment outcomes were categorized as endoscopic and clinical. Endoscopic outcomes included en block resection, complete resection, and endoscopic success. En bloc resection was defined as resection in a one-piece fashion with no residual tumor viewed endoscopically. Complete resection was defined as resection in the absence of any remnant lesion or recurrence in control biopsies at the end of the study regardless of the number of treatment sessions.6,10 Endoscopic success was defined as total removal via excision, irrespective of the number of sessions required for removal, and absence of recurrence or recurrence during the follow-up period, treated sufficiently by EP.11 Clinical outcomes included duration of antiprotease injection during the hospital stay, transfusion requirement, length of hospital stay, mortality, and recurrence.
The pre-procedural diagnosis of ampullary adenoma was obtained for each patient by forceps biopsy. Endoscopic ultrasound (EUS), transpapillary intraductal ultrasound (IDUS), ERCP, and computed tomography or magnetic resonance image were applied for the assessment of the extent of the tumor.
An indication for EP was based on EUS, IDUS, and ERCP findings that showed that the tumor had not infiltrated the bile or pancreatic ducts and that the adenoma or cancer invasion was confined to the mucosa.6,10 Patients with invasive carcinoma, metastatic disease, coagulation abnormalities, or severe concomitant disorders were not indicated for ampullectomy. Patients with ampullary adenoma in familiar adenomatous polyposis were excluded. Written informed consent was obtained from all patients. This study was approved by the Institutional Review Board of Yonsei University College of Medicine, Korea.
EP was performed by five expert endoscopists with patients under sedation. The entire procedure was carried out under fluoroscopic guidance using a side-view duodenoscope (TJF 260; Olympus, Tokyo, Japan). EP was performed using one of the following methods: 1) snare papillectomy or 2) endoscopic mucosal resection (EMR). For en block resection, we performed EP by grasping the adenoma at the base with a standard polypectomy snare and applying an Endocut current effect 2 using an ERBE generator. The EMR was performed for only periampullary portion, because the submucosal injection of papilla can also make the lesion difficult to capture using a snare.12 Small, remnant lesions unamenable to snare resection were either removed by endoscopic forceps or fulgurated by argon plasma coagulation (APC; ERBE Elektromedizin, Germany). The placement of a pancreatic stent was routinely performed. The application of biductal sphincterotomy or biliary stent was individualized in each case.
As EP and biliary or pancreatic sphincterotomy are higher-risk procedures for bleeding, we weighted the benefit of discontinuation of anti-platelets (aspirin and non-steroidal anti-inflammatory drugs) against the risk of thromboembolic events. We discontinued aspirin and/or non-steroidal anti-inflammatory drugs for 5 to 7 days before the procedure, depending on the underlying indication for antiplatelet therapy.13 We used antiprotease including nafamostat and gabexate mesylate: 50 mg of nafamostat was infused intravenously twice a day and 600 mg of gabexate mesylate intravenously once a day.
We classified the macroscopic type of lesion as an exposed type vs. an unexposed type, based on a previous macroscopic classification of carcinoma of the ampulla of Vater.7 We defined the exposed type of lesion as polypoid tumors protruding through the papilla into the duodenum (periampullary type). The unexposed type was defined as polypoid tumors of the common channel without a duodenal luminal component (intraampullary type), or a mixed form of periampullary and intraampullary type.
Resected specimens were diagnosed according to the revised Vienna classification.14 Histologic grading was classified as low-grade dysplasia (LGD), high-grade dysplasia (HGD), differentiated adenocarcinoma, and undifferentiated adenocarcinoma. The positivity of vertical and lateral cuts of the resected specimen was examined to confirm complete endoscopic resection.
Complications were classified into early (pancreatitis, bleeding, perforation, and others) or late (post-papillectomy stenosis) onset. EP-related pancreatitis was defined according to consensus criteria.15 It was diagnosed if there was an elevation in pancreatic enzymes of at least three times the upper limit of the normal range 24 hours after the procedure, new onset of pain in the upper abdomen, and hospitalization for at least 2 days. The grade of pancreatitis severity was also determined according to consensus guidelines; mild EP-related pancreatitis resulting in a hospitalization of less than or equal to 3 days; moderate EP-related pancreatitis resulting in a hospitalization of 4 to 10 days; and severe EP-related pancreatitis resulting in a hospitalization of 11 or more days, leading to the development of pancreatic necrosis or pseudocyst, or requiring percutaneous or surgical intervention. Bleeding was diagnosed if there was clinical or endoscopical evidence of bleeding after completion of the procedure. The clinical or endoscopic evidence comprised the following: 1) fresh hematemesis or fresh blood passing from the nasogastric tube; 2) passage of fresh melena or hematochezia with evidence of bleeding (a systolic blood pressure <100 mm Hg, a heart rate >100 bpm, or a drop in hemoglobin level of >2 g/dL within 24 h); and 3) bleeding demonstrated by endoscopy.
Follow-up endoscopy with forceps biopsy was scheduled after 3 to 6 months and at yearly intervals thereafter. A second endoscopy was not performed routinely. If the resected lesion recurred and additional endoscopic treatment was possible, endoscopic treatment was performed every 2 to 3 months until there was no residual adenoma, with surveillance every 6 to 12 months for the next 2 years according to the recommendations.16 When the patient had a small lesion with tubular adenoma, the interval of endoscopic surveillance was increased to 2 or 3 years gradually. Surgical resection was done if additional endoscopic treatment was not feasible.
Statistical analysis included the chi-square test, Fisher's exact test, and Mann-Whitney U test. Results were presented as proportions, or median with range (minimum-maximum) as indicated. Kaplan-Meier's test and log-rank test were performed for the evaluation of recurrence rate and comparison of recurrence rate between two groups, respectively. A p value <0.05 was considered statistically significant. Statistical analysis was performed using the SAS program (version 9.2, SAS Institute, Cary, NC, USA).
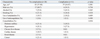
This study included 39 patients (23 men and 16 women; median age 62.0 years, range 34.0-88.0 years). There were no significant differences in baseline characteristics, including age, gender, and the use of antiplatelets, anticoagulation, and comorbidities, between the no complication group (n=28) and the complication group (n=11) (Table 1). There was no significant difference in the distribution of five endoscopists between the two groups (p=0.074).
Clinically, the presence of symptoms, including pancreatitis prior to EP was similar in the two groups. In laboratory characteristics, amylase (median 111 IU/L vs. 361 IU/L, p=0.006) and lipase levels (median 67 IU/L vs. 457 IU/L, p=0.007), 24 hours after EP, were significantly higher in the complication group than in the no complication group. However, amylase and lipase levels within 24 hours after EP and other laboratory serologic values, including hemoglobin levels, white blood cell count, platelet count, aspartate transaminase, and alanine transaminase levels, total bilirubin, and albumin levels were not significantly different between the two groups.
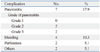
The complications of EP are summarized in Table 2. Fifteen complications from 11 patients (28.2%) occurred, and all were early complications. The most common complication was EP-related pancreatitis (n=7, 17.9%), followed by bleeding (n=4, 10.3%) and perforation (n=2, 5.1%). Seven EP-related cases of pancreatitis were composed of grade 2 (n=5) and grade 3 (n=2) pancreatitis. They were successfully managed with medical treatment, including fasting, intravenous hydration, and antiprotease injection. No necrotizing pancreatitis or pancreatic pseudocysts developed during EP-related pancreatitis. Four patients experienced procedure-related bleeding, which was managed with endoscopic clips, electric coagulation, or APC. Two patients with perforations were managed with endoscopic clips and medical treatments. Two patients developed pneumonia after the procedure, which was managed without further complications. No cholangitis or biliary stenosis occurred. None of the patients underwent any surgeries for complication management.
We analyzed the endoscopic and histologic characteristics of the two groups (Table 3). Endoscopic characteristics including the size and type of lesion, placement of pancreatic or biliary duct stent after EP, performance of biductal sphincterotomy, and resection method (snare papillectomy vs. EMR) did not reveal any significant differences. The sizes and lengths of stents ranged from 5 to 8 Fr and from 5 to 7 cm, respectively. The histologic diagnosis of resected specimens was not significantly different between the two groups (p=0.541), however, was associated with complete resection rate. The complete resection rate in LGD (n=24), HGD (n=7), adenocarcinoma (n=3), and benign tissue (n=5) was 83.3%, 42.9%, 33.3%, and 100%, respectively (p=0.032). Thus, it appears that as the tumor progressed from adenoma to cancer, the complete resection rate decreased.
The overall en block resection rate, complete resection rate, and endoscopic success rate were 89.7%, 74.4%, and 92.3%, respectively. The overall median value of the duration of antiprotease injection during the hospital stay and length of hospital stay were 3.0 days (range 1.0-18.0 days) and 8.0 days (range 4.0-24.0 days), respectively. Thirty-two of the enrolled patients (82.1%) received anti-protease agents. There was no mortality in the present study. Treatment outcomes of the two groups are listed in Table 4. The en block resection rate, complete resection rate, and endoscopic success rate did not differ significantly between the two groups. Clinically, the duration of antiprotease injection during the hospital stay was significantly longer (median 2.0 days vs. 5.0 days, p=0.017) and the transfusion requirements were significantly higher (0% vs. 27.3%, p=0.018) in the complication group than the no complication group. The hospital stay tended to be longer (median 6.5 days vs. 9.0 days, p=0.083) in the complication group. During a median follow-up period of 15 months (range 1-25 months), three lesions (10.3%) recurred among patients with complete resection (n=29) and five lesions (12.8%) recurred among enrolled patients. In three HGD lesions and two LGD lesions, recurrence occurred. However, the histology at the time of recurrence sometimes differed from the prior histology of the resected specimen. In one patient with previously resected HGD, LGD occurred. In another patient with resected LGD, adenocarcinoma occurred. In the latter case, a pathological examination revealed that the resection margin was clear at the time of ampullectomy, but pancreaticoduodenectomy was performed 22 months later, because of the occurrence of adenocarcinoma. Additionally, the recurrence was not associated with the initial size of the lesion, as the lesions without recurrence and with recurrence did not show a significant difference in lesion size (p=0.281).
The present study focused on the procedure-related complications and long-term treatment outcomes of EP. Previous EP studies reported complication rates that ranged from 8% to 32%,8,9 similar to 28.2% among enrolled patients in our study. Complications included EP-related pancreatitis, bleeding, and perforation as early complications, and pancreatic duct stenosis as a late complication.
Acute pancreatitis is the most common major complication of ERCP, accounting for substantial morbidity and occasional death.17,18 In the present study, EP-related pancreatitis occurred in 17.9% of patients, presenting mainly as moderate to severe, and accompanying a prolonged hospital stay, whereas EP-related pancreatitis occurred in 5% to 15% of patients, mainly as a mild form as seen in a previous study.10 To predict the possible occurrence of EP-related pancreatitis, clinicians routinely measure amylase and lipase levels, usually within 24 hours of EP. However, a transient elevation of amylase and lipase levels often occurs after EP. To differentiate such a transient elevation of amylase and lipase levels from EP-related pancreatitis, we serially tested amylase and lipase levels within 24 hours as well as 24 hours after EP. Significant differences in amylase and lipase levels between the two groups were noted only 24 hours after EP. Therefore, we recommend a follow-up measurement of amylase and lipase levels 24 hours after EP to predict EP-related pancreatitis in patients with significant abdominal pain.
Currently, the preventive effects of a pancreatic stent for EP-related pancreatitis are not well established.6,19 The only prospective, randomized, controlled trial evaluating the role of a prophylactic pancreatic stent for the reduction of EP-related pancreatitis showed a statistically significant decrease in the rate of EP-related pancreatitis in the stent group.20 In our study, we placed the pancreatic stent in 64.1% of patients, and found that the occurrence of EP-related pancreatitis between unstented (n=14) and stented lesions (n=25) was not significantly different (14.3% vs. 20.0%, p=1.000). A large-scale study is needed to confirm the protective effect of pancreatic stent for pancreatitis after EP.
The submucosal injection may help predict the presence of malignancy and reduce the risk of bleeding and perforation.8 In our study, submucosal injection was performed in 38.5% of patients. Four bleeding complications and two perforation complications occurred, but only in cases without submucosal injection. Lesions without submucosal injection (n=24) tended to have more bleeding and perforation complications than lesions with submucosal injection (n=15) (25% vs. 0%, p=0.065). Thus, submucosal injections during EP seem to reduce the risk of bleeding and perforation.
Histologic diagnosis of resected specimens was associated with a statistical difference in complete resection but not with complications in a previous study.21 Our study showed that as the tumor progressed from LGD to adenocarcinoma, the complete resection rate decreased. We attribute this interesting finding to an underestimation of the extent of the malignant margin, caused by endoscopist's prejudice based on histology using forceps biopsy with poor sensitivity.5,22 Additionally, the biology of the tumor might affect the complete resection rate. Due to unique location of the tumor and associated complications, it is difficult to resect the tumor with sufficient margins, thus missing lateral and vertical spread of the tumor. Therefore, in cases with HGD and adenocarcinoma, endoscopists should evaluate previously undiagnosed intraductal extension using a cholangiogram and a pancreaticogram immediately prior to resection and try to resect the tumor with as much a margin as possible.
Complications of EP in terms of treatment outcomes have not been evaluated previously. Our study showed that the occurrence of complications might not change the endoscopic treatment outcomes. In contrast, the complications affected the clinical outcomes, including a longer duration of antiprotease injection during the hospital stay and a more required transfusion. In addition, the complication group tended to have a longer hospital stay than the no complication group. We made the decision to discharge patients after EP, based on symptoms, signs, and other evidence including laboratory evaluation. We had a policy of early discharge after EP, mainly due to the medical cost burden on patients, however, the heterogenous composition of five endoscopists might have affected individualized decisions for discharge and the length of hospital stay in the present study.
The recurrence rate in the present study was 10.3% among patients with complete resection and 12.8% among enrolled patients during a median follow up of 15 months, similar to earlier studies, which ranged from 2.8% to 15%.7,10 The occurrence of complications may not change the recurrence rate.
Interestingly, one patient with progressive recurrence was noted in this study. The histology of completely resected specimen was LGD, and the histology of surgically resected specimen of the recurred lesion 22 months after EP was differentiated adenocarcinoma that invaded the duodenum and distal pancreatic duct. This is the first report of progressive recurrence in ampullary adenoma, though malignant recurrence from possibly remnant malignant foci in the ampullary adenoma cannot be excluded. Although progressive recurrence is very rare, clinicians should be aware of the possibility of malignant foci in the ampullary adenoma when recurrent lesion shows progressive histology from LGD to HGD. Even if the histology of the resected specimen was LGD, careful and regular follow up is still needed.
This study had some limitations. First, this is a single-center, retrospective study. Second, the number of enrolled patients was small, because of the rarity of disease. Third, heterogeneity in resection methods is also a possible limitation. In addition, because the complication group included heterogenous complications, our data may not represent the safety and outcome of a specific complication.
In spite of these limitations, the present study had some important messages. This is the first study investigating the clinical, endoscopic, and histologic characteristics of complications after EP. In addition, this is the first study of the clinical implications of complications after EP in terms of treatment outcomes and recurrence pattern.
In conclusion, if symptoms are present, amylase and lipase levels 24 hours after EP could help predict the possibility of EP-related pancreatitis. Histologic diagnosis with resected specimens may be associated with complete resection. Patients with complications need longer duration of antiprotease injection during their hospital stay and more transfusions. The recurrence rate is not significantly high in completely resected cases, nevertheless, there is a possibility of progressive recurrence. Further studies are needed to reduce the complication rate of EP and increase the complete resection rate at the same time.
Figures and Tables
Table 1
Baseline Characteristics of 39 Patients Underwent Endoscopic Papillectomy for Ampullary Adenoma
References
1. Rosenberg J, Welch JP, Pyrtek LJ, Walker M, Trowbridge P. Benign villous adenomas of the ampulla of Vater. Cancer. 1986; 58:1563–1568.
2. Arvanitis ML, Jagelman DG, Fazio VW, Lavery IC, McGannon E. Mortality in patients with familial adenomatous polyposis. Dis Colon Rectum. 1990; 33:639–642.

3. Stolte M, Pscherer C. Adenoma-carcinoma sequence in the papilla of Vater. Scand J Gastroenterol. 1996; 31:376–382.

4. Seifert E, Schulte F, Stolte M. Adenoma and carcinoma of the duodenum and papilla of Vater: a clinicopathologic study. Am J Gastroenterol. 1992; 87:37–42.
5. Yamaguchi K, Enjoji M, Kitamura K. Endoscopic biopsy has limited accuracy in diagnosis of ampullary tumors. Gastrointest Endosc. 1990; 36:588–592.

7. Yamao T, Isomoto H, Kohno S, Mizuta Y, Yamakawa M, Nakao K, et al. Endoscopic snare papillectomy with biliary and pancreatic stent placement for tumors of the major duodenal papilla. Surg Endosc. 2010; 24:119–124.

8. Desilets DJ, Dy RM, Ku PM, Hanson BL, Elton E, Mattia A, et al. Endoscopic management of tumors of the major duodenal papilla: refined techniques to improve outcome and avoid complications. Gastrointest Endosc. 2001; 54:202–208.

9. Norton ID, Gostout CJ, Baron TH, Geller A, Petersen BT, Wiersema MJ. Safety and outcome of endoscopic snare excision of the major duodenal papilla. Gastrointest Endosc. 2002; 56:239–243.

10. Bohnacker S, Soehendra N, Maguchi H, Chung JB, Howell DA. Endoscopic resection of benign tumors of the papilla of vater. Endoscopy. 2006; 38:521–525.

11. Patel R, Davitte J, Varadarajulu S, Wilcox CM. Endoscopic resection of ampullary adenomas: complications and outcomes. Dig Dis Sci. 2011; 56:3235–3240.

12. Boix J, Lorenzo-Zúñiga V, Moreno de Vega V, Domènech E, Gassull MA. Endoscopic resection of ampullary tumors: 12-year review of 21 cases. Surg Endosc. 2009; 23:45–49.

13. Anderson MA, Ben-Menachem T, Gan SI, Appalaneni V, Banerjee S, et al. ASGE Standards of Practice Committee. Management of antithrombotic agents for endoscopic procedures. Gastrointest Endosc. 2009; 70:1060–1070.

15. Cotton PB, Lehman G, Vennes J, Geenen JE, Russell RC, Meyers WC, et al. Endoscopic sphincterotomy complications and their management: an attempt at consensus. Gastrointest Endosc. 1991; 37:383–393.

16. Catalano MF, Linder JD, Chak A, Sivak MV Jr, Raijman I, Geenen JE, et al. Endoscopic management of adenoma of the major duodenal papilla. Gastrointest Endosc. 2004; 59:225–232.

17. Freeman ML, Guda NM. Prevention of post-ERCP pancreatitis: a comprehensive review. Gastrointest Endosc. 2004; 59:845–864.

18. Elmunzer BJ, Scheiman JM, Lehman GA, Chak A, Mosler P, Higgins PD, et al. A randomized trial of rectal indomethacin to prevent post-ERCP pancreatitis. N Engl J Med. 2012; 366:1414–1422.

19. Han J, Kim MH. Endoscopic papillectomy for adenomas of the major duodenal papilla (with video). Gastrointest Endosc. 2006; 63:292–301.

20. Harewood GC, Pochron NL, Gostout CJ. Prospective, randomized, controlled trial of prophylactic pancreatic stent placement for endoscopic snare excision of the duodenal ampulla. Gastrointest Endosc. 2005; 62:367–370.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download