Abstract
Purpose
To identify the effect of insufficient lymph node dissection (LND) on the survival of patients with pT2 gastric cancer.
Materials and Methods
A total of 340 patients (120 patients with insufficient LND and others with D2 LND) who underwent gastrectomy for pT2 gastric cancer between January 2008 and December 2010 were included.
Results
The incidence of preoperatively diagnosed early gastric cancer was higher and there were fewer metastatic lymph nodes (LNs) in the insufficient LND group than the D2 group, but there was no survival difference between two groups (p=0.365). Among the 89 patients with metastatic LNs after D2 LND, 13 patients (14.6%) had metastatic LNs at selected N2 stations (#10, 11, or 12a), but none of these patients were in the pN1 category. One patient had five metastatic LNs at station #11p with no metastatic LNs at any other stations. The number of metastatic LNs was identified as the only risk factor for LN metastasis at selected N2 stations by logistic regression.
Surgery is the only method for curing advanced gastric cancer (AGC).1 The depth of tumor invasion,1,2 the metastatic lymph node (LN) status, and R0 resection are the most important independent prognostic factors for gastric cancer (GC).3,4 Therefore, adequate LN dissection (LND) during gastrectomy is vital for both accurate tumor staging and achieving R0 resection during GC treatment.
Although there have long been debates about the necessity of D2 LND for GC,5-8 D2 LND is considered a standard procedure in GC surgery.9 A recent guideline recommended that gastrectomy with D1+ LND is sufficient for cases of early gastric cancer (EGC), and D2 LND should be performed for AGC.10 When the tumor depth is indicative of EGC in the preoperative evaluation, surgeons usually perform less than D2 LND. However, sometimes patients who were considered to have EGC through preoperative evaluation can turn out to have AGC in the final pathological results after surgery, and most of these patients (54.4-68.9%) were discovered to have cancer that was invading the proper muscle layer (pT2).11,12
When considering the possibility of remaining metastatic LNs and tumor downstaging, it would be insufficient to perform anything less than D2 LND in pT2 GC. Therefore, surgeons worry about the surgical adequacy and oncological effects of less than D2 LND, the insufficient LND, in pT2 GC. In the present study, we evaluated the oncological safety and surgical curability of insufficient LND in pT2 GC.
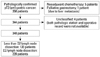
The medical records of patients who underwent gastrectomy for GC at Yonsei University Severance Hospital between January 2008 and December 2010 were reviewed, and 350 patients were confirmed to have pT2 GC in their final pathologic reports. Among them, 10 patients were excluded for the following reasons: neo-adjuvant chemotherapy in 5 patients, palliative gastrectomy in 1 patient, and unclear medical records in 4 patients (Fig. 1).
The standard extent of LND for gastric cancer in our institution is D1+ for EGC, and D2 for AGC. D2 LND was defined according to Japanese GC guidelines 201010,13 as follows. LND at stations #1, 3, 4sb, 4d, 5, 6, 7, 8a, 9, 11p, and 12a was classified as D2 LND in distal gastrectomy, but if the surgeon skipped any station among #11p and/or 12a, then the dissection was defined as insufficient LND. For total gastrectomy, LND at stations #1, 2, 3, 4, 5, 6, 7, 8a, 9, 10, 11p, 11d, and 12a was categorized as D2 LND, but if the surgeon skipped any station among #10, 11d, and/or 12a, then the dissection was classified as insufficient LND.
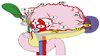
Stations #7, 8a, 9, 10, 11, and 12a were considered extragastric LNs, or N2 stations. In this study, stations #10, 11, and 12a were designated as selected N2 stations (Fig. 2), because LND of stations #7, 8a, and 9 is routinely performed in all GC surgeries with curative intent.
According to Japanese guidelines for gastric cancer surgery,10 all 340 patients who were finally diagnosed as pT2 GC from our institution should undergo D2 LND. However, 120 patients underwent insufficient LND due to the patients' conditions and tumor underestimation in the preoperative work-up.
We retrospectively studied clinicopathological factors such as age, sex, body mass index (BMI), various comorbidities, and preoperative diagnosis; perioperative factors including resection extent, approach methods, operative time, amount of blood loss, transfusion, postoperative hospital stay, and postoperative complications; and pathologic factors such as number of metastatic and retrieved LNs, tumor size, Borrmann type, Lauren classification, histologic grade, and pN category. To evaluate the preoperative diagnosis of gastric cancer, we reviewed radiologic reports of computed tomography (CT). If we found any comment about wall thickening in the gastric wall, it was determined to be AGC otherwise we consider it EGC. In addition, the presence of detectable lymph nodes was recorded.
Laparoscopic or laparoscopic-assisted gastrectomy and robotic or robotic-assisted gastrectomy were considered minimally invasive surgeries (MISs). After gastrectomy, most patients were treated with adjuvant chemotherapy when necessary according to the patient's final pathologic stages. Papillary and well to moderate differentiated tubular adenocarcinomas were classified as differentiated types; and mucinous carcinoma, signet-ring cell carcinoma, and poorly differentiated tubular adenocarcinoma were classified as undifferentiated types. Pathological stage evaluation was based on the seventh edition of the International Union Against Cancer Classification.14
Any death, regardless of cause, was recorded and reflected in the overall survival (OS) during follow-up. To elucidate the risk factors of LN metastasis at selected N2 stations (the difference of the extent of LND between two groups), the data of patients with information about metastasis at each LN station were analyzed. In addition, the pattern of recurrence after gastrectomy in each group was evaluated.
For continuous variables, independent t-tests were used, and chi-square tests or Fisher's exact tests were adopted for dichotomous variables as appropriate. A binary logistic regression model was applied to calculate the odds ratio (OR) of LN metastasis at selected N2 station, and the OR of performing insufficient LND. The Kaplan-Meier method was used to report survival curves and estimate the mean survival and the 95% confidence intervals (CI). To assess the difference in survival between the two groups, the log rank test and Tarone-Ware test were applied. In addition, Cox proportional hazard model was applied with and without adjustment to estimate hazard ratios (HR). In all cases, a p-value of less than 0.05 was considered a rejection of the null hypothesis. The statistical analyses were performed using IBM SPSS 20.0 (SPSS Inc., Chicago, IL, USA), and the Kaplan-Meier graph was created using R version 2.13.0 (R Foundation for Statistical Computing, Vienna, Austria).
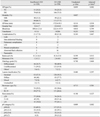
The demographics of the patients were similar between the two groups (Table 1). The mean age of patients was approximately 59 years in both groups, and the mean BMI was approximately 23 kg/m2. There were no significant differences in the distribution of the type of comorbidities, American Society of Anesthesiologists score, and previous history of abdominal surgery. Insufficient LND was associated with the preoperative diagnosis. When a patient was diagnosed with EGC by preoperative endoscopy, the OR was 3.0 (p<0.001), and if CT was used to diagnose EGC, the OR of performing insufficient LND was 2.5 (p=0.01). In addition, LNs were more frequently detected from CT scans in D2 LND than insufficient LND (p=0.021).
The perioperative outcomes are presented in Table 2. There were 41 cases (34.2%) of total gastrectomy in the insufficient LND group and 54 cases (24.5%) in the D2 LND group. MISs were more frequently performed in the insufficient LND group than in the D2 LND group (33.3% vs. 22.3%, p=0.027). The operative time (180.3 min vs. 172.8 min), the incidence of transfusion (3.4% vs. 6.8%), and the incidence of postoperative complications were similar between the two groups. The length of hospital stay (9.0 days vs. 10.4 days) and the amount of blood loss (109.6 mL vs. 122.5 mL) appeared to be higher in the D2 LND group than in the insufficient LND group, but the differences did not reach statistical significance after adjusting for age, sex, and operative modality (p=0.309 and 0.799, respectively).
The distribution of microscopic and macroscopic findings of tumors, and tumor size showed no difference between the two groups. The number of retrieved LNs was also similar between the two groups regardless of the extent of gastrectomy and the modality (open/MIS). The number of metastatic LNs was greater in the D2 LND group than in the insufficient LND group (1.2 vs. 2.4, p=0.022), and consequently, the pN category was higher in the D2 LND group than in the insufficient LND group (p=0.002).
After gastrectomy, no in-hospital mortality was identified, and the median follow-up duration was 31 months for OS and 29.5 months for recurrence. During follow-up, 22 patients died (7 patients in the insufficient LND group and 15 patients in the D2 LND group); among them, 14 patients died because of GC, and the other patients died from other causes such as pneumonia, sudden cardiac arrest, and other cancers. Five patients were identified to have recurrence in the insufficient LND group as well as 23 patients in the D2 LND group - detailed recurrence patterns are shown in Table 3. The mean survival duration of the insufficient LND group was 51.2 months (95% CI: 49.2-53.2), compared with 52.0 months in the D2 LND group (95% CI: 50.6-53.5). The total OS was not different between the two groups (log rank test p-value: 0.946, Tarone-Ware test p-value: 0.811) (Fig. 3). In addition, there was no statistical difference between the two groups when analyzed according to pN category (Fig. 4). In Cox regression analysis, the unadjusted HR for OS after insufficient LND was 0.969 (p=0.946). After adjustment for age, sex, and pN category, the HR increased to 1.56 (95% CI: 0.60-4.08), but this value was not statistically significant (p=0.366). The result was similar when the HR was adjusted by pN category and preoperative diagnosis based on computed tomography and esophagogastroduodenoscopy (HR: 1.72, 0.64-4.65, p=0.282). In terms of disease free survival, the HR adjusted by age, sex, and pN category was 0.82 (95% CI: 0.29-2.30, p=0.709).
Among the 103 patients with metastatic LNs after D2 LND, precise information regarding the presence of metastasis at each LN station was available for 89 patients. Thirteen of these patients (14.6%) had any metastatic LN at selected N2 stations (#10, 11, or 12a). The characteristics of these patients are described in Table 4. All of the 103 patients had more than two metastatic lymph nodes (pN2-3 category), and no patient was in the pN1 category (pN2 category n=3; pN3 category n=10). Only one patient had metastatic LNs at station #10, and six and seven patients had metastatic LNs at stations #11 and #12a, respectively. One patient had metastatic LNs at both station #11 and #12a. One patient had five metastatic LNs at station #11p with no metastasis at other stations after distal gastrectomy. Logistic regression failed to reveal any risk factors for LN metastasis at the selected N2 stations excluding the number of metastatic LNs (p<0.001) (Table 5).
Although the diagnostic modalities and the accuracy of preoperative diagnosis for GC have been improved, the diagnostic accuracy for the depth of tumor invasion was reported as approximately 70-85%.15-18 Focal invasion of the tumor is considered to be one possible cause of underestimated tumor depth in preoperative evaluation, and the incidence of focal invasion in pT2 GC was reported at 32.1%.11 Therefore, preoperative EGC is occasionally diagnosed as AGC after surgical resection, whereas the opposite is observed in some cases. Because radical gastrectomy with D2 LND is considered a standard surgery for AGC, we evaluated the oncologic outcome and safety margin of insufficient LND in pT2 GC.
In the present study, there was no LN metastasis at selected N2 stations (#10, 11, or 12a) after D2 LND, which are not removed during insufficient LND, when the final pathologic results revealed pN0 or pN1 category. In other words, even if insufficient LND was performed in patients with pT2 GC, it appears to be a secure operation when the final pathological reports revealed pT2N0 and pT2N1 disease. Moreover, there were no differences in survival and recurrence between the patients who underwent insufficient LND and those who underwent D2 LND in the pN0 and pN1 categories.
The reason for skipped LN metastasis is not fully understood, and the possible theories are as follows: 1) there may be remaining occult metastases that are undetected by pathologic examination; 2) the minor omentum has many lymphatic routes; 3) there may be only a few perigastric LNs19; and 4) although the location is extragastric, it is the first LN in the lymphatic drain system.20 In our data, among the 89 patients with metastatic LNs after D2 LND, one patient (1.1%) displayed skipped LN metastasis at station #11p after distal gastrectomy and this patients had pN2 category (five metastatic LNs).
Among patients in the pN2 and pN3 category, 9.4% (3/32) and 34.5% (10/29) of patients, respectively, had metastatic LNs at selected N2 stations (#10, 11, or 12a). If these 13 patients had undergone insufficient LND, the metastatic LNs at the selected N2 stations would have remained after surgery. In these patients, insufficient LND cannot be considered adequate or R0 resection. However, there was no statistical difference in OS between the two groups among patients in the pN2 and pN3 category.
Reoperation or chemotherapy could be recommended if elucidation of the risk factors of LN metastasis at selected N2 stations in patients who underwent insufficient LND is possible. However, our data did not reveal any risk factors of LN metastasis at selected N2 stations, excluding the number of metastatic LNs. Higher number of metastatic LNs was found to be associated with a higher risk of metastasis at selected N2 stations in the pN2, and pN3 category.
The major limitation of the present study is the study design. The retrospective design can create selection bias. Lower mean number of metastatic LNs in the insufficient LND group would represent a selection bias, which means the baseline characteristics of the insufficient LND group could be better than D2 LND group even though both group share same pT stage. In addition, no difference between OS and recurrence between two groups is unexpected. Even though the subgroup analyses by pN category also showed same results, the different baseline characteristics would affect this result. The difference of dissected stations between the two groups varied only 1-3 (#11p and/or #12a for distal gastrectomy and #10 and/or #11d and/or #12a) and the retrieved LNs were similar in the two groups. Finally, the median follow up period was not over the line of 36 months. Since a 15 year follow up randomized controlled trial of disease specific survival between D1 and D2 LND showed an only 11% benefit for D2 LND,8 it is difficult to make concrete conclusions from this study.
The authors of several recent reports discussed the risk of LN metastasis at extragastric regions, but they did not focus on selected N2 stations (#10, 11, or 12a) or pT2 GC.21,22 Currently, it appears to be nearly impossible to determine whether insufficient LND for pT2 GC is secure for patients with pN2 or pN3 category after gastrectomy. In addition, the postoperative diagnosis will be not useful to prevent insufficient LND in clinical practice. Therefore, surgeons should perform D2 LND whenever there is any intraoperative suspicion of LNs metastasis or pathological diagnosis, even if the lesion was diagnosed as EGC in the preoperative evaluation.
In conclusion, insufficient LND in pT2 GC appears to be secure when the final pathologic results reveal to be in the pN0 and pN1 category. If the final pathologic results are in the pN2 and pN3 category, surgeons should carefully monitor the patient during follow-up because of the high possibility of remaining metastatic LNs at selected N2 stations.
Figures and Tables
Table 1
Patient Demographics

LND, lymph node dissection; BMI, body mass index; ASA, American Society of Anesthesiologists; HTN, hypertension; DM, diabetes mellitus; CVA, cerebrovascular accident; EGC, early gastric cancer; AGC, advanced gastric cancer; EGD, esophagogastroduodenoscopy; CT, computed tomography; LN, lymph node; GC, gastric cancer; SD, standard deviation.
*Data were not available for some patients who were diagnosed with GC in other hospitals.
ACKNOWLEDGEMENTS
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2011-0011301).
The authors thank Mr. Dong-Su Jang, Medical Illustrator, Department Research Affairs, Yonsei University College of Medicine, for his help with the figures.
References
1. Nakamura K, Ueyama T, Yao T, Xuan ZX, Ambe K, Adachi Y, et al. Pathology and prognosis of gastric carcinoma. Findings in 10,000 patients who underwent primary gastrectomy. Cancer. 1992; 70:1030–1037.

2. Adachi Y, Mori M, Maehara Y, Sugimachi K. Long-term survival after resection for advanced gastric carcinoma. J Clin Gastroenterol. 1995; 21:208–210.

3. Roukos DH. Current status and future perspectives in gastric cancer management. Cancer Treat Rev. 2000; 26:243–255.

4. Siewert JR, Böttcher K, Stein HJ, Roder JD. Relevant prognostic factors in gastric cancer: ten-year results of the German Gastric Cancer Study. Ann Surg. 1998; 228:449–461.
5. Memon MA, Subramanya MS, Khan S, Hossain MB, Osland E, Memon B. Meta-analysis of D1 versus D2 gastrectomy for gastric adenocarcinoma. Ann Surg. 2011; 253:900–911.

6. Cuschieri A, Weeden S, Fielding J, Bancewicz J, Craven J, Joypaul V, et al. Surgical Co-operative Group. Patient survival after D1 and D2 resections for gastric cancer: long-term results of the MRC randomized surgical trial. Br J Cancer. 1999; 79:1522–1530.

7. Robertson CS, Chung SC, Woods SD, Griffin SM, Raimes SA, Lau JT, et al. A prospective randomized trial comparing R1 subtotal gastrectomy with R3 total gastrectomy for antral cancer. Ann Surg. 1994; 220:176–182.

8. Songun I, Putter H, Kranenbarg EM, Sasako M, van de Velde CJ. Surgical treatment of gastric cancer: 15-year follow-up results of the randomised nationwide Dutch D1D2 trial. Lancet Oncol. 2010; 11:439–449.

9. Lee JH, Kim KM, Cheong JH, Noh SH. Current management and future strategies of gastric cancer. Yonsei Med J. 2012; 53:248–257.

10. Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2010 (ver. 3). Gastric Cancer. 2011; 14:113–123.
11. Lee SE, Lee JH, Kook MC, Yoo JS, Ryu KW, Lee JS, et al. T2 or greater disease but were diagnosed preoperatively with early gastric cancer. Hepatogastroenterology. 2008; 55:2282–2286.
12. Park DJ, Han SU, Hyung WJ, Kim MC, Kim W, Ryu SY, et al. Long-term outcomes after laparoscopy-assisted gastrectomy for advanced gastric cancer: a large-scale multicenter retrospective study. Surg Endosc. 2012; 26:1548–1553.

13. Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer. 2011; 14:101–112.
14. Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A. AJCC Cancer Staging Handbook. 7th ed. New York: Springer;2010.
15. Puli SR, Batapati Krishna, Bechtold ML, Antillon MR, Ibdah JA. How good is endoscopic ultrasound for TNM staging of gastric cancers? A meta-analysis and systematic review. World J Gastroenterol. 2008; 14:4011–4019.

16. Habermann CR, Weiss F, Riecken R, Honarpisheh H, Bohnacker S, Staedtler C, et al. Preoperative staging of gastric adenocarcinoma: comparison of helical CT and endoscopic US. Radiology. 2004; 230:465–471.

17. Cardoso R, Coburn N, Seevaratnam R, Sutradhar R, Lourenco LG, Mahar A, et al. A systematic review and meta-analysis of the utility of EUS for preoperative staging for gastric cancer. Gastric Cancer. 2012; 15:Suppl 1. S19–S26.

18. Furukawa K, Miyahara R, Itoh A, Ohmiya N, Hirooka Y, Mori K, et al. Diagnosis of the invasion depth of gastric cancer using MDCT with virtual gastroscopy: comparison with staging with endoscopic ultrasound. AJR Am J Roentgenol. 2011; 197:867–875.

19. Kosaka T, Ueshige N, Sugaya J, Nakano Y, Akiyama T, Tomita F, et al. Lymphatic routes of the stomach demonstrated by gastric carcinomas with solitary lymph node metastasis. Surg Today. 1999; 29:695–700.

20. Kitagawa Y, Fujii H, Mukai M, Kubota T, Ando N, Watanabe M, et al. The role of the sentinel lymph node in gastrointestinal cancer. Surg Clin North Am. 2000; 80:1799–1809.





 PDF
PDF ePub
ePub Citation
Citation Print
Print






 XML Download
XML Download