Abstract
Purpose
The aim of this study was to investigate the differences of expression in glycolysis-related proteins such as Glut-1, carbonic anhydrase (CA) IX, and monocarboxylate transporter (MCT) 4 according to the myoepithelial cell (MEC) and basement membrane (BM) status in solid papillary carcinoma (SPC) of the breast.
Materials and Methods
Immunohistochemical evaluation of Glut-1, CAIX, and MCT4, as well as p63 and type IV collagen, were performed on 23 SPC cases.
Results
Six and nine cases of SPC showed the presence and absence of myoepithelial cells, respectively, and eight cases belonged to the borderline status (p63-positive MEC on some areas of the outer tumor surface but not in others). BM was partially or completely absent in 14 cases and present in nine cases. SPC lacking BM more frequently showed high expression of CAIX than SPC with BM (p=0.037).
Solid papillary carcinoma (SPC) of the breast, which was first described by Maluf and Koerner,1 occurs mainly in elderly women and was considered as a variant of ductal carcinoma in situ (DCIS) in its first description. Histologically, SPC is composed of circumscribed, solid nodules of tumor cells, which are typically ovoid and spindle shaped, with intervening network of fibrovascular cores but without a discrete papillary structure.1 The tumor cells can exhibit neuroendocrine features, including granular eosinophilic cytoplasm and fine nuclear chromatin, as well as immunohistochemical expression of chromogranin and synaptophysin.1,2 Though SPC was considered as a variant of DCIS when first described, it has subsequently been suggested that SPC showing a lack of myoepithelial cells (MEC) in the periphery of a tumor nodule might instead be a subset of invasive carcinoma with pushing margin.2,3 Additionally, the possibility of SPC as an invasive carcinoma is further supported by the occurrence of regional lymph node metastasis in SPC.2
Although there are a number of possible processes involved in the progression from DCIS to invasive carcinoma during breast cancer tumorigenesis, Gatenby and Gillies4 have described the metabolic process as a cellular adaptation with three phases: hypoxia, glycolysis, and acidosis. Regional hypoxia occurs when tumor cells move away from the basement membrane owing to cellular proliferation within a duct, consequently inducing an adaptive upregulation of glycolysis. Due to intracellular acidosis caused by glycolysis with lactate production, the tumor cells require resistance to acid-induced toxicity. Upregulation of glycolysis and subsequent cellular resistance to acid, which together confer a powerful adaptive advantage, allow for unlimited proliferation of tumor cells and eventually function as selection forces in the transition process from a preinvasive lesion to an invasive cancer. During glycolysis in tumor cells, the Glut-1 protein is critical for glucose uptake into cells,5 and the carbonic anhydrase (CA) IX enzyme has an important role in the elimination of glycolysis-related acidosis, regulating pH by reverse hydration of carbon dioxide to carbonic acid.6 Additionally, monocarboxylate transporter (MCT) 4 plays a role in the efflux of lactate produced by glycolysis out of the tumor cells.7
We hypothesize that there may be some differences in glycolysis and/or acid resistance between SPCs with and without invasion. The aim of this study was to investigate differences in glycolysis and acid resistance according to MEC and basement membrane (BM) status in SPC using immunohistochemical detection of Glut-1 and CAIX.
This study included patients undergoing surgical resection of SPC at Severance Hospital from January 2005 to December 2011. All of the cases were retrospectively reviewed by breast pathologists (Kwon JE, Koo JS). SPC was defined histologically as follows: circumscribed, solid nodules of tumor cells, which are typically ovoid and spindle shaped, with an intervening network of fibrovascular cores but without a discrete papillary structure.1 When extracellular mucin was observed in SPC-like tumors, we diagnosed mucinous carcinoma if tumor cells floated on mucin pools. Otherwise, we defined it as SPC. Cases in which other types of carcinoma were identified were excluded. A total of 37 cases of SPC were collected from the pathology department. Fourteen cases which were mixed type tumors were excluded from the study; mixed with mucinous carcinoma in 8 cases and with usual invasive ductal carcinoma in 6 cases. Histological examination was performed using H&E-stained slides. The histological grade was assessed using the Nottingham grading system.8 This study was approved by the Institutional Review Board (IRB) of Yonsei University Severance Hospital. All experiments were performed in compliance with institutional guidelines. Informed consents from patients were exempt by IRB.
The antibodies used for immunohistochemistry in this study are shown in Table 1. All immunostains were performed using formalin-fixed, paraffin-embedded tissue sections. Using a microtome, 5-µm-thick sections were obtained, transferred to adhesive slides, and dried at 62℃ for 30 minutes. The sections were then placed in a glass jar with 10 mM citrate buffer (pH 6.0) and irradiated in a microwave oven for 15 min. The sections were allowed to cool in the jar at room temperature for 20 min. The slides were then rinsed with Tris buffered saline (TBS). A blocking reagent was added for 10 min after quenching the endogenous peroxidase activity in 0.3% hydrogen peroxide for 10 min. After incubation with the primary antibodies, immunodetection was performed with biotinylated antimouse immunoglobulin, followed by peroxidase-labeled streptavidin using a labeled streptavidin biotin kit with 3,3'-diaminobenzidine chromogen as substrate. Optimal primary antibody incubation time and concentration were determined via serial dilution for each immunohistochemical assay with an identically fixed and embedded tissue block.
All immunohistochemical markers were assessed using light microscopy. Pathologic parameters such as estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor 2 (HER-2) status were obtained from patient report. A cut-off value of 1% positively stained nuclei was used to define ER and PR positivity.9 HER-2 staining was analyzed according to the guidelines of the American Society of Clinical Oncology/College of American Pathologists using the following categories: 0=no immunostaining; 1+=weak incomplete membranous staining, less than 10% of tumor cells; 2+=complete membranous staining, either uniform or weak in at least 10% of tumor cells; and 3+=uniform intense membranous staining in at least 30% of tumor cells. HER-2 immunostaining was considered positive when strong (3+) membranous staining was observed, whereas cases with 0 to 1+ were regarded as negative.10 The cases showing 2+ HER-2 expression were evaluated for HER-2 amplification by fluorescent in situ hybridization.
Immunohistochemical staining results for Glut-1, CAIX, MCT4 were defined by the proportion of positively stained cells as follows: negative=no staining; low positive=less than 50% of tumor cells stained; high positive=50% or more of tumor cells stained. Chromogranin A and synaptophysin was considered positive when more than 10% of tumor cells were expressed. MEC status at the outer surface of SPC was evaluated by p63 immunostaining. Using normal duct or acini in the background as positive control, the presence of p63-positive MEC covering the entire outer surface was defined as "MEC present," and the absence of p63-positive MEC covering the entire outer surface as "MEC absent." When p63-positive MEC were found on some areas of the outer tumor surface but not in others, it was defined as "MEC indefinite." To identify the presence of BM, immunohistochemical staining for type IV collagen was used. With the positive control of normal duct or acini in the background, clear linear expression of type IV collagen on the outer surface of the SPC was considered BM positive, and otherwise was considered BM negative.
Data were processed using SPSS for Windows, version 12.0 (SPSS Inc., Chicago, IL, USA). Student's t and Fisher's exact tests were used to evaluate differences in continuous and categorical variables, respectively. Statistical significance was defined as a p-value less than 0.05.
A total of 23 patients were included in the study (Table 2). The mean age of enrolled patients was 57.1±13.4 years, and the mean size of tumor was 1.6±0.5 cm. All cases were ER-positive, and 19 cases (82.6%) were also PR-positive. Immunostaining for HER-2 was negative in 21 cases, and no amplification was detected in either of the two cases with 2+ immunopositivity for HER-2 by fluorescence in situ hybridization. Mucin production was noted in 7 cases (30.4%). Sentinel lymph node examination was performed in 10 out of 23 patients; none of the cases showed lymph node involvement or distant metastasis. There was no difference in clinicopathologic characteristics according to combined MEC and BM status.
The absence of MEC on the outer surface was correlated with negative type IV collagen (p=0.036). Among 6 cases with MEC on the outer surface, 5 (83.3%) cases were positive for type IV collagen. Out of 9 cases without MEC on the outer surface, 7 (77.8%) cases were negative for type IV collagen.
Both MEC (+)/BM (-) and MEC (-)/BM (-) cases had higher CAIX positivity than MEC (+)/BM (+) and MEC (-)/BM (+) cases, although these were not statistically significant differences (p=0.059) (Table 3).
There was no significant difference in other factors between SPC with MEC at the outer surface and SPC without. Stromal MCT4 expression was noted only in p63-negative or indefinite cases (Table 4).
This study investigated the expression of glycolysis-related protein in SPC categorized by MEC and/or BM status as markers of invasiveness. As to the invasiveness in SPC, there have been inconsistencies in previous studies, some of which reported the absence of MEC in SPC,1,3 and others of which reported the presence of MEC in SPC.11
In the present study, the presence of MEC at the outer surface of SPC, labeled by p63 immunostaining, was significantly correlated with type IV collagen status (p=0.036). In other words, if the outer surface of SPC lacked MEC, BM was also absent, suggesting that, in SPC, alteration of MEC is accompanied by the loss of BM. On the other hand, there was a small subset of SPC with only one positive feature, either MEC (-)/BM (+) or MEC (+)/BM (-). This finding is consistent with previous reports of DCIS in which intact BM was identified in spite of a loss of MEC,12,13 as well as other reports of invasive ductal carcinoma which secreted a BM-like material around the tumor.14,15 Alternatively, it is possible that the p63 and type IV collagen antibodies used in this study failed to detect MEC and BM, respectively.
We show that the loss of type IV collagen expression was correlated with high expression of CAIX, irrespective of p63 expression (p=0.032). CAIX is a transmembrane glycoprotein which is involved in the reverse hydration of carbon dioxide into carbonic acid, thus regulating pH. Its main role is to neutralize the intracellular acidic microenvironment which is caused by lactic acid produced by aerobic glycolysis in tumor cells.6 Because there was no significant difference in Glut-1 expression depending on the expression of type IV collagen, it is hard to postulate that increased expression of CAIX in SPC is directly associated with glycolysis. It has also been suggested that other pathways such as phosphatidylinositol-3-kinase pathway16 and mitogen-activated protein kinase pathway17 are also involved in the regulation of CAIX expression.
The loss of type IV collagen in SPCs with high level of CAIX expression could also result from the increased extracellular acidity. The CAIX-mediated efflux of H+ out of the cells to neutralize the intracellular acidic microenvironment would lead to extracellular acidity, which has been reported to degrade the extracellular matrix and BM.18 Therefore, as an acid-resistant phenotype, SPC with high expression of CAIX may show more invasive growth and tumor progression. Further investigation is required to determine whether SPC with a high level of CAIX expression shows increased tumor aggressiveness, because most SPC is known to have an indolent clinical course.
This study exhibited that MCT4 was expressed in stromal cells rather than tumor cells only in SPC with indefinite or negative MECs. MCT4 is a protein transferring the lactate produced by glycolysis out of the cells.7 MCT4 expression in the stroma of breast carcinoma has been reported in the literature and stromal MCT4 expression was described as a marker of oxidative stress in cancer-associated fibroblasts.19 Although it is unclear whether stromal expression of MCT4 in SPC represent cancer-associated oxidative stress, our result of MCT4 expression in the stromal fibroblasts only in the cases with myoepithelial alteration suggests the role of epithelial-stromal interaction in the process of invasion. Further study is required to elucidate the mechanism of the stromal MCT4 expression and its implication.
In conclusion, in SPCs, there was no significant difference in the expression of Glut-1 depending on the invasiveness. But, cases with BM loss, the CAIX expression were increased. Increased CAIX level, possibly associated with acquisition of acid-resistance of the tumor cells, may lead to extracellular acidity and subsequent BM degradation.
Figures and Tables
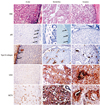 | Fig. 1Expression of glycolysis-related proteins according to myoepithelial cell and basement membrane status. In situ SPC shows p63-positive myoepithelial cells in the outer surface of the tumor nodule (arrow) and a basement membrane that is positive for type IV collagen (arrow). Indefinite SPC shows p63 negativity on one side and p63-positive myoepithelial cells at the outer surface of the other side of the tumor nodule and is negative for type IV collagen. In invasive SPC, both p63 and type IV are negative at the outer surface of the tumor nodule. SPC which is negative for type IV collagen shows a high level of CAIX expression, and invasive carcinoma shows high level of MCT4 expression in the stroma (S) as well as tumor cells (T). SPC, solid papillary carcinoma; MCT, monocarboxylate transporter; CAIX, carbonic anhydrase IX. |
Table 2
Patient Characteristics According to Combined Myoepithelial Cell and Basement Membrane Status

Table 3
Expression of Glycolysis-Related Proteins According to Combined Myoepithelial Cell and Basement Membrane Status
ACKNOWLEDGEMENTS
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2012R1A1A1002886).
References
1. Maluf HM, Koerner FC. Solid papillary carcinoma of the breast. A form of intraductal carcinoma with endocrine differentiation frequently associated with mucinous carcinoma. Am J Surg Pathol. 1995; 19:1237–1244.
2. Nassar H, Qureshi H, Adsay NV, Visscher D. Clinicopathologic analysis of solid papillary carcinoma of the breast and associated invasive carcinomas. Am J Surg Pathol. 2006; 30:501–507.

3. Dickersin GR, Maluf HM, Koerner FC. Solid papillary carcinoma of breast: an ultrastructural study. Ultrastruct Pathol. 1997; 21:153–161.

4. Gatenby RA, Gillies RJ. Why do cancers have high aerobic glycolysis? Nat Rev Cancer. 2004; 4:891–899.

5. Clavo AC, Brown RS, Wahl RL. Fluorodeoxyglucose uptake in human cancer cell lines is increased by hypoxia. J Nucl Med. 1995; 36:1625–1632.
6. Pastorek J, Pastoreková S, Callebaut I, Mornon JP, Zelník V, Opavský R, et al. Cloning and characterization of MN, a human tumor-associated protein with a domain homologous to carbonic anhydrase and a putative helix-loop-helix DNA binding segment. Oncogene. 1994; 9:2877–2888.
7. Halestrap AP, Price NT. The proton-linked monocarboxylate transporter (MCT) family: structure, function and regulation. Biochem J. 1999; 343 Pt 2:281–299.

8. Elston CW, Ellis IO. Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology. 1991; 19:403–410.

9. Hammond ME, Hayes DF, Dowsett M, Allred DC, Hagerty KL, Badve S, et al. American Society of Clinical Oncology/College Of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Clin Oncol. 2010; 28:2784–2795.

10. Wolff AC, Hammond ME, Schwartz JN, Hagerty KL, Allred DC, Cote RJ, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J Clin Oncol. 2007; 25:118–145.

11. Tsang WY, Chan JK. Endocrine ductal carcinoma in situ (E-DCIS) of the breast: a form of low-grade DCIS with distinctive clinicopathologic and biologic characteristics. Am J Surg Pathol. 1996; 20:921–943.
12. Tramm T, Kim JY, Tavassoli FA. Diminished number or complete loss of myoepithelial cells associated with metaplastic and neoplastic apocrine lesions of the breast. Am J Surg Pathol. 2011; 35:202–211.

13. Tsubura A, Shikata N, Inui T, Morii S, Hatano T, Oikawa T, et al. Immunohistochemical localization of myoepithelial cells and basement membrane in normal, benign and malignant human breast lesions. Virchows Arch A Pathol Anat Histopathol. 1988; 413:133–139.

14. Gusterson BA, Warburton MJ, Mitchell D, Ellison M, Neville AM, Rudland PS. Distribution of myoepithelial cells and basement membrane proteins in the normal breast and in benign and malignant breast diseases. Cancer Res. 1982; 42:4763–4770.
15. Wetzels RH, Holland R, van Haelst UJ, Lane EB, Leigh IM, Ramaekers FC. Detection of basement membrane components and basal cell keratin 14 in noninvasive and invasive carcinomas of the breast. Am J Pathol. 1989; 134:571–579.
16. Kaluz S, Kaluzová M, Chrastina A, Olive PL, Pastoreková S, Pastorek J, et al. Lowered oxygen tension induces expression of the hypoxia marker MN/carbonic anhydrase IX in the absence of hypoxia-inducible factor 1 alpha stabilization: a role for phosphatidylinositol 3'-kinase. Cancer Res. 2002; 62:4469–4477.
17. Kopacek J, Barathova M, Dequiedt F, Sepelakova J, Kettmann R, Pastorek J, et al. MAPK pathway contributes to density- and hypoxia-induced expression of the tumor-associated carbonic anhydrase IX. Biochim Biophys Acta. 2005; 1729:41–49.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download