Abstract
Primary prevention and early detection of cardiovascular disease is important, as it is the leading cause of death throughout world. Risk stratification algorithms, such as Framingham Risk Score and European Systematic Coronary Risk Evaluation, that utilize a combination of various traditional risk factors have been developed to improve primary prevention. However, the accuracy of these algorithms for screening high risk patients is moderate at best. Accordingly, the use of biomarkers or imaging studies may improve risk stratification. Carotid ultrasound, which measures both carotid intima-media thichkness (cIMT) and carotid plaque, is useful in detecting the degree of subclinical carotid atherosclerosis, and has the advantage of being noninvasive and safe. Several large epidemiologic studies have indicated that cIMT and carotid plaque are closely related with other cardiovascular risk factors and may be useful for risk reclassification in subjects deemed to be at intermediate risk by traditional risk scores. Moreover, recent clinical guidelines for management of hypertension or dyslipidemia highlight the usefulness of cIMT in high risk patients. In this article, we review evidence for the usefulness of measurement of cIMT and carotid plaque for cardiovascular risk stratification.
Cardiovascular disease is the leading cause of mortality throughout the world, with studies showing cardiovascular disease to be the cause of death in 30% of all mortalities reported in the USA.1 Atherosclerosis is a disease process that begins at an early age and is progressive in nature. Atherosclerotic lesions do not arouse any symptoms in the early stage, but its initial presentation may result in catastrophic cardiovascular events resulting from plaque rupture.2 As such, it is imperative to identify subjects at increased risk of cardiovascular disease and modify their risk factors early on. Also, the treatment of advanced atherosclerosis is less effective than the inhibition of atherosclerosis progression.3 However, traditional risk factors for predicting cardiovascular disease, such as the Framingham Risk Score (FRS) and the European Systematic Coronary Risk Evaluation, comprise modest predictive value for future cardiovascular events.4 For this reason, the treatment of atherosclerosis at an earlier stage is crucial with more precise patient selection for preventive treatment. Landmark studies have demonstrated that measurements of subclinical atherosclerosis, such as carotid ultrasound, ankle brachial index and coronary calcium score, offer significant benefit in improving cardiovascular risk prediction beyond that with traditional risk factors.5,6,7 Among these measurements, ultrasonographic measurement of carotid intima-media thickness (cIMT) and carotid plaque have been widely applied to detect early atherosclerotic lesions. This review aims to discuss up to date evidence for the clinical usefulness of cIMT and plaque measurement for cardiovascular risk stratification.
In spite of recent advances in treatment strategies, the incidence of cardiovascular disease is expected to increase as a result of ageing populations.8 In addition, the socioeconomic cost thereof is high due to the chronic nature of cardiovascular disease. Therefore, the establishment of a prevention plan is essential, and research regarding biomarkers, imaging studies, and risk classification models for predicting cardiovascular events have been studied extensively.
Although FRS is the standard score system for predicting cardiovascular risk using traditional risk factors, it is modest at best in predicting cardiovascular risk. This is not surprising since studies have shown that the majority of patients with cardiovascular disease would have been classified as low risk by traditional risk scores. A 26-year follow-up data of the Framingham Heart Study revealed that 35% of subjects with total cholesterol level below 200 mg/dL, which is considered a desirable cholesterol level, were predisposed to cardiovascular disease. Moreover, the total cholesterol levels were the same in 80% of individuals who experienced myocardial infarction versus those who did not experience the event.9 The National Cholesterol Education Program Adult Treatment Panel III (NCEP-ATPIII) guidelines, which are based on FRS, suggest that the target goal of low density lipoprotein (LDL) cholesterol lowering, the primary target for reduction of cardiovascular disease, differs according to risk category, as determined using traditional cardiovascular risk factors.10 However, some studies have revealed some limitations with the NCEP-ATPIII guidelines on risk stratification. Akosah, et al. showed that the only 25% of young patients with acute myocardial infarction would have been recommended for statin drug therapy criteria according to NCEP-ATPIII guidelines.11 In addition, this guideline did not categorize 69% of acute myocardial infarction patients to the drug therapy group in a large cohort without a history of coronary artery disease.12
High blood pressure is one of the most important causes of cardiovascular disease and there is no argument that treatment of hypertension lowers cardiovascular risk. However, in the British United Provident Association (BUPA) study, which included 21520 subjects of ages 35-64 years, blood pressure was not a strong indicator for prediction of future cardiovascular events. The study demonstrated that the distribution of blood pressures among men who died of ischemic heart disease and men who did not exhibited noticeable overlap.13
Risk factors, like those above, are closely related with the incidence and the progression of atherosclerosis. However, these are indirect rather than direct markers for the current status of atherosclerosis and have limitations for evaluating atherosclerosis. Thus, more direct markers with anatomical delineation through imaging technology or functional studies have been developed and widely used. Among them, many researchers or clinicians have utilized cIMT measurement via ultrasonography.
Carotid ultrasonography can measure cIMT and detect focal atherosclerotic plaque using ultrasound. The measurement of cIMT has several advantages for monitoring of atherosclerosis. First, cIMT can be performed with no adverse effects on subjects. Second, cIMT can be carried out at relatively low cost. Third, cIMT gives better visualization of atherosclerotic changes on arterial wall than other imaging modalities. In addition, ultrasound B-mode imaging of cIMT has been shown to be well correlated with IMT measured on microscopic examination.14 Therefore, the measurement of cIMT can provide precise information about atherosclerotic burden. Data from several sources have identified that traditional risk prediction models could not accurately reflect the presence of atherosclerosis as measured by carotid ultrasonography. Naqvi, et al. studied the prevalence of subclinical atherosclerosis in the groups of low, intermediate, and high FRS in 136 asymptomatic subjects using carotid ultrasonography. In the 103 low-risk (FRS<10%) subjects, 66% had cIMT>75th percentile or plaque >1.5 mm.15 In another study, when carotid ultrasonography was performed in 336 asymptomatic healthy young subjects with FRS of <5%, nearly 38% had high risk carotid ultrasound findings, defined as the cIMT>75th percentile. These studies demonstrated that traditional risk factors do not accurately reflect the actual plaque burden in the arteries, and as such, cIMT may be of use for risk stratification.16 The recently published European Society of Hypertension/European Society of Cardiology have defined either a mean cIMT of over 0.9 mm or the presence of carotid plaque as a marker of target organ damage in hypertension.17
Studies have demonstrated significant correlations between the degree of cIMT with other cardiovascular risk factors. Age and high blood pressure are known to be major determinants of cIMT in the general population.18 This is consistent with the fact that atherosclerosis progresses with age and high blood pressure. Moreover, other proatherogenic factors, like total cholesterol, LDL-cholesterol and insulin resistance, have been shown to be significantly associated with cIMT.19,20 The Bougalusa Heart Study showed that the progression of cIMT was significantly associated with fasting blood glucose and systolic blood pressure in young adults.21 When the lifetime risk for cardiovascular disease was estimated using an algorithm including total cholesterol, blood pressure, smoking, and diabetes mellitus (DM), individuals with higher life time risk experienced a greater subclinical atherosclerosis burden.22 These associations between traditional risk factors for cardiovascular disease and cIMT suggest that cIMT exhibits potential as a surrogate marker for predicting cardiovascular events.
Many prospective studies have evaluated the relationship between cIMT and cardiovascular clinical events.23 Although each study set a differing cutoff point of high risk, the studies demonstrated an absolute yearly risk for cardiovascular disease, ranging from 1.6% to 3.2%, with increase in cIMT. Additionally, the relative risk of high cIMT ranged from 2.2 to 3.2 for coronary heart disease.24,25,26 The Atherosclerosis Risk In Communities (ARIC) study, which was an epidemiologic study of cardiovascular incidence in the general population, revealed that a higher coronary heart disease incidence was significantly associated with the both the increasing degree of cIMT and the presence of plaque (Fig. 1).27 Baldassarre, et al. demonstrated that the progression rate of maximum cIMT was significantly associated with cardiovascular risk.28 The results from the aforementioned epidemiologic studies clearly demonstrated that cIMT is associated with increased cardiovascular risk.
Numerous studies have demonstrated that cIMT is a significantly predictive for cardiovascular events. However, what is still not centain is the additional predictive value of cIMT beyond traditional risk score. Lorenz, et al. studied the additive value of cIMT using the 10-year follow-up data of 4904 subjects from the Carotid Atherosclerosis Progression Study (CAPS).29 Subjects were reclassified to new risk strata using traditional risk prediction models with cIMT, and were compared to classifications based on traditional risk scores to determine the improvement in cardiovascular risk prediction with the addition of cIMT. Surprisingly, the risk prediction with cIMT did not demonstrate an improvement in cardiovascular risk prediction, compared to the traditional risk prediction model. However, a study done in 13415 subjects enrolled in the ARIC study showed that adding cIMT to traditional risk factors improves predictability. Also, this study showed that the addition of carotid plaque further improved the prediction strength.27 Likewise, Polak, et al. demonstrated that cIMT is able to contribute to improving traditional risk prediction models.5 They carried out a study in 2965 subjects of the Framingham Offspring Study Cohort and found that the prediction of cardiovascular disease became better after addition of maximum intima-media thickness of internal carotid artery, with an improvement of the net reclassification index by 7.6% (p<0.001); however, no improvement in net reclassification was demonstrated when mean intima-media thickness of common carotid artery was added to the traditional risk factors. In this study, the presence of plaque, defined as internal cIMT of more than 1.5 mm, was associated with significant increase in both C statistics [0.014, 95% confidence interval (CI): 0.003-0.025, p=0.02] and improvement in the net reclassification index by 7.3% (p<0.01). Also, the presence of plaque significantly improved the prediction of new-onset cardiovascular disease (Fig. 2). Therefore, the assessment of both common carotid and internal carotid plaque add incremental value in predicting cardiovascular events.
Notwithstanding, cIMT may not be useful for risk stratification in all spectrums of risk categories. Among 10-year follow-up results from the CAPS, which included a cohort of relatively young and low risk subjects (n=4904) in a primary healthcare setting, cIMT did not improve the risk classification of individuals.29 Likewise, a recent meta-analysis of 14 population cohorts of 45828 subjects by Den Ruijter, et al. demonstrated that addition of common cIMT to FRS was associated with a small improvement in 10-year risk prediction of first time myocardial infarction or stroke, but without significant improvement in the net reclassification (0.8%, 95% CI: 0.1-1.6%). However, the net reclassification improvement was 3.6% (95% CI: 2.7-4.6%) in subjects with intermediate risk of cardiovascular disease.30 Therefore, cIMT may be useful for risk stratification in subjects with intermediate risk of cardiovascular disease as determined by traditional risk factors.
With cIMT being accepted as a measure of subclinical atherosclerosis and organ damage, it has been presumed that the progression of cIMT, as a marker of atherosclerosis progression, will be associated with adverse cardiovascular prognosis. However, recent publications are contradictory to these assumptions. Analysis of the European Lacidipine Study on Atherosclerosis showed that both baseline IMT and on-treatment IMT, but not treatment induced changes, were associated with incident cardiovascular events in treated hypertensive patients.31 In the PROG-IMT collaborative project, a meta-analysis was done on 16 studies with 36984 participants who had at least two measurements of cIMT and outcome data regarding myocardial infarction, stroke, and death.32 The results showed that for mean cIMT progression the hazard ratio was not significantly increased (hazard ratio=0.98, 95% CI: 0.95-1.01) when adjusted for age, gender, mean cIMT, and vascular risk factors. As such, recent European Society of Hypertension (ESH) and European Society of Cardiology (ESC) guidelines did not endorse the usefulness of follow-up cIMT for assessing vascular organ damage in hypertensive subjects.17 From the evidence available at this time, it is uncertain as to whether or not change in cIMT during the follow-up carotid ultrasound is associated with increased risk of cardiovascular disease. As such, follow-up carotid ultrasound should not be performed for the sole purpose of cardiovascular risk stratification.
Despite the widespread use of cIMT, it still has limitations. Above all, the threshold value of cIMT, above which the risk is increased, needs to be clearly defined. cIMT is affected by multiple factors. Age is the most influential factor on cIMT and common cIMT increases 0.01 mm annually.33 Thus, it is hard to apply one absolute value to the general population as abnormal IMT. Although the ESH/ESC guidelines define a high risk cIMT finding as a mean cIMT of more than 0.9 mm, this may not be generalizable to all populations, especially Asians. In addition, as cIMT is a continuous variable, a definition to distinguish between plaque and vessel wall hypertrophy is arbitrary. The 34th Bethesda conference suggested the use of nomograms or ratios of observed to predicted IMT based on age and other factors affecting cIMT, such as the approximate age-adjusted 75th percentile values for cIMT.34 The Manheim cIMT Consensus defines plaque as a focal structure encroaching into the arterial lumen by at least 0.5 mm or 50% of the surrounding wall or demonstrates a plaque thickness of >1.5 mm.35 However, each of these studies still used an arbitrary cutoff point for abnormal IMT, and more research is needed to further standardize a protocol.
Another key question that needs to be resolved is how the presence of high risk cIMT findings in a patient affects management decisions. When abnormal results are found on carotid ultrasound screening, physicians are more likely to prescribe aspirin and lipid-lowering medication.36 However, recent meta-analyses suggested that aspirin may not offer benefits for primary prevention of cardiovascular disease when considering the increase in bleeding risk.37,38 Therefore, a prospective randomized study to determine whether the addition of antiplatelet agents, based on the presence of high risk cIMT finding alone, may have significant benefit needs to be performed. Also, while several clinical trials reported that statin reduced the progression of carotid atherosclerosis and cardiovascular disease risk, there are no prospective studies to show the cardiovascular benefit of prescribing statin based on the presence of high risk cIMT. Although the ESC/EAS guidelines for the management of dyslipidemia grouped subjects with carotid plaque as very high risk and recommended an LDL-cholesterol treatment goal of 70 mg/dL in these patients, this is based more on expert recommendation rather than clinical evidence.39 As such, prospective randomized study to determine the LDL-cholesterol treatment goal for obtaining maximal efficacy in subjects whose risk classification has changed according to cIMT needs to be performed. In conclusion, although there are still unresolved issues regarding the usefulness of cIMT in primary prevention, cIMT is considered to have additive value in cardiovascular risk prediction, especially in subjects with intermediate risk of cardiovascular disease by traditional risk factors. However, further studies are needed to determine treatment strategies to possibly reduce cardiovascular risk in patients with high risk cIMT.
Figures and Tables
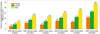 | Fig. 1The coronary heart disease incidence rate according to various carotid intima-media thickness (cIMT) categories with and without the presence of plaque. For the overall group (green bar), men (yellow bar), or women (orange bar), the higher cIMT and the presence of plaque is associated with a higher incidence of coronary heart disease (Adapted from Nambi, et al. J Am Coll Cardiol 2010;55:1600-7, with permission from Elsevier).27
|
 | Fig. 2Kaplan-Meier estimates of new-onset cardiovascular disease (CVD) according to the presence of plaque. The 8-year rates of cardiovascular disease increase with the presence of internal carotid artery plaque for participants overall (A) and for each Framingham Risk Score category: low risk (0 to <6%) (B), intermediate risk (6 to 20%) (C), and high risk (>20%) (D) (Adapted from Polak, et al. N Engl J Med 2011;365:213-21, with permission from Massachusetts Medical Society).5
|
ACKNOWLEDGEMENTS
This work was supported by a grant of the Korean Health Technology R&D Project, Ministry of Health & Welfare, Republic of Korea (HI13C0715).
References
1. Anderson RN, Smith BL. Deaths: leading causes for 2002. Natl Vital Stat Rep. 2005; 53:1–89.
2. Giroud D, Li JM, Urban P, Meier B, Rutishauer W. Relation of the site of acute myocardial infarction to the most severe coronary arterial stenosis at prior angiography. Am J Cardiol. 1992; 69:729–732.

3. Insull W Jr. The pathology of atherosclerosis: plaque development and plaque responses to medical treatment. Am J Med. 2009; 122:1 Suppl. S3–S14.

4. Batsis JA, Lopez-Jimenez F. Cardiovascular risk assessment--from individual risk prediction to estimation of global risk and change in risk in the population. BMC Med. 2010; 8:29.
5. Polak JF, Pencina MJ, Pencina KM, O'Donnell CJ, Wolf PA, D'Agostino RB Sr. Carotid-wall intima-media thickness and cardiovascular events. N Engl J Med. 2011; 365:213–221.

6. Lee AJ, Price JF, Russell MJ, Smith FB, van Wijk MC, Fowkes FG. Improved prediction of fatal myocardial infarction using the ankle brachial index in addition to conventional risk factors: the Edinburgh Artery Study. Circulation. 2004; 110:3075–3080.

7. Polonsky TS, McClelland RL, Jorgensen NW, Bild DE, Burke GL, Guerci AD, et al. Coronary artery calcium score and risk classification for coronary heart disease prediction. JAMA. 2010; 303:1610–1616.

8. Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006; 3:e442.

9. Castelli WP. Lipids, risk factors and ischaemic heart disease. Atherosclerosis. 1996; 124:Suppl. S1–S9.

10. Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). JAMA. 2001; 285:2486–2497.
11. Akosah KO, Schaper A, Cogbill C, Schoenfeld P. Preventing myocardial infarction in the young adult in the first place: how do the National Cholesterol Education Panel III guidelines perform? J Am Coll Cardiol. 2003; 41:1475–1479.

12. Yoon YE, Rivera JJ, Kwon DA, Chae IH, Jeong MH, Rha SW, et al. National Cholesterol Education Panel III guidelines performance role in preventing myocardial infarction in a large cohort without a history of coronary artery disease: Korea Acute Myocardial Infarction Registry study. Prev Cardiol. 2009; 12:109–113.

13. Law M, Wald N, Morris J. Lowering blood pressure to prevent myocardial infarction and stroke: a new preventive strategy. Health Technol Assess. 2003; 7:1–94.

14. Pignoli P, Tremoli E, Poli A, Oreste P, Paoletti R. Intimal plus medial thickness of the arterial wall: a direct measurement with ultrasound imaging. Circulation. 1986; 74:1399–1406.

15. Naqvi TZ, Mendoza F, Rafii F, Gransar H, Guerra M, Lepor N, et al. High prevalence of ultrasound detected carotid atherosclerosis in subjects with low Framingham risk score: potential implications for screening for subclinical atherosclerosis. J Am Soc Echocardiogr. 2010; 23:809–815.

16. Eleid MF, Lester SJ, Wiedenbeck TL, Patel SD, Appleton CP, Nelson MR, et al. Carotid ultrasound identifies high risk subclinical atherosclerosis in adults with low framingham risk scores. J Am Soc Echocardiogr. 2010; 23:802–808.

17. Mancia G, Fagard R, Narkiewicz K, Redon J, Zanchetti A, Böhm M, et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J. 2013; 34:2159–2219.
18. Prati P, Vanuzzo D, Casaroli M, Di Chiara A, De Biasi F, Feruglio GA, et al. Prevalence and determinants of carotid atherosclerosis in a general population. Stroke. 1992; 23:1705–1711.

19. Davis PH, Dawson JD, Riley WA, Lauer RM. Carotid intimal-medial thickness is related to cardiovascular risk factors measured from childhood through middle age: The Muscatine Study. Circulation. 2001; 104:2815–2819.

20. Pauletto P, Palatini P, Da Ros S, Pagliara V, Santipolo N, Baccillieri S, et al. Factors underlying the increase in carotid intima-media thickness in borderline hypertensives. Arterioscler Thromb Vasc Biol. 1999; 19:1231–1237.

21. Johnson HM, Douglas PS, Srinivasan SR, Bond MG, Tang R, Li S, et al. Predictors of carotid intima-media thickness progression in young adults: the Bogalusa Heart Study. Stroke. 2007; 38:900–905.

22. Berry JD, Liu K, Folsom AR, Lewis CE, Carr JJ, Polak JF, et al. Prevalence and progression of subclinical atherosclerosis in younger adults with low short-term but high lifetime estimated risk for cardiovascular disease: the coronary artery risk development in young adults study and multi-ethnic study of atherosclerosis. Circulation. 2009; 119:382–389.

23. Lorenz MW, Markus HS, Bots ML, Rosvall M, Sitzer M. Prediction of clinical cardiovascular events with carotid intima-media thickness: a systematic review and meta-analysis. Circulation. 2007; 115:459–467.

24. Salonen JT, Salonen R. Ultrasonographically assessed carotid morphology and the risk of coronary heart disease. Arterioscler Thromb. 1991; 11:1245–1249.

25. O'Leary DH, Polak JF, Kronmal RA, Manolio TA, Burke GL, Wolfson SK Jr. Cardiovascular Health Study Collaborative Research Group. Carotid-artery intima and media thickness as a risk factor for myocardial infarction and stroke in older adults. N Engl J Med. 1999; 340:14–22.
26. Lorenz MW, von Kegler S, Steinmetz H, Markus HS, Sitzer M. Carotid intima-media thickening indicates a higher vascular risk across a wide age range: prospective data from the Carotid Atherosclerosis Progression Study (CAPS). Stroke. 2006; 37:87–92.

27. Nambi V, Chambless L, Folsom AR, He M, Hu Y, Mosley T, et al. Carotid intima-media thickness and presence or absence of plaque improves prediction of coronary heart disease risk: the ARIC (Atherosclerosis Risk In Communities) study. J Am Coll Cardiol. 2010; 55:1600–1607.

28. Baldassarre D, Veglia F, Hamsten A, Humphries SE, Rauramaa R, de Faire U, et al. Progression of carotid intima-media thickness as predictor of vascular events: results from the IMPROVE study. Arterioscler Thromb Vasc Biol. 2013; 33:2273–2279.

29. Lorenz MW, Schaefer C, Steinmetz H, Sitzer M. Is carotid intima media thickness useful for individual prediction of cardiovascular risk? Ten-year results from the Carotid Atherosclerosis Progression Study (CAPS). Eur Heart J. 2010; 31:2041–2048.

30. Den Ruijter HM, Peters SA, Anderson TJ, Britton AR, Dekker JM, Eijkemans MJ, et al. Common carotid intima-media thickness measurements in cardiovascular risk prediction: a meta-analysis. JAMA. 2012; 308:796–803.

31. Zanchetti A, Hennig M, Hollweck R, Bond G, Tang R, Cuspidi C, et al. Baseline values but not treatment-induced changes in carotid intima-media thickness predict incident cardiovascular events in treated hypertensive patients: findings in the European Lacidipine Study on Atherosclerosis (ELSA). Circulation. 2009; 120:1084–1090.

32. Lorenz MW, Polak JF, Kavousi M, Mathiesen EB, Volzke H, Tuomainen TP, et al. Carotid intima-media thickness progression to predict cardiovascular events in the general population (the PROG-IMT collaborative project): a meta-analysis of individual participant data. Lancet. 2012; 379:2053–2062.

33. Howard G, Sharrett AR, Heiss G, Evans GW, Chambless LE, Riley WA, et al. Carotid artery intimal-medial thickness distribution in general populations as evaluated by B-mode ultrasound. ARIC Investigators. Stroke. 1993; 24:1297–1304.

34. Redberg RF, Vogel RA, Criqui MH, Herrington DM, Lima JA, Roman MJ. 34th Bethesda Conference: Task force #3--What is the spectrum of current and emerging techniques for the noninvasive measurement of atherosclerosis? J Am Coll Cardiol. 2003; 41:1886–1898.

35. Touboul PJ, Hennerici MG, Meairs S, Adams H, Amarenco P, Bornstein N, et al. Mannheim carotid intima-media thickness and plaque consensus (2004-2006-2011). An update on behalf of the advisory board of the 3rd, 4th and 5th watching the risk symposia, at the 13th, 15th and 20th European Stroke Conferences, Mannheim, Germany, 2004, Brussels, Belgium, 2006, and Hamburg, Germany, 2011. Cerebrovasc Dis. 2012; 34:290–296.

36. Johnson HM, Turke TL, Grossklaus M, Dall T, Carimi S, Koenig LM, et al. Effects of an office-based carotid ultrasound screening intervention. J Am Soc Echocardiogr. 2011; 24:738–747.

37. Seshasai SR, Wijesuriya S, Sivakumaran R, Nethercott S, Erqou S, Sattar N, et al. Effect of aspirin on vascular and nonvascular outcomes: meta-analysis of randomized controlled trials. Arch Intern Med. 2012; 172:209–216.

38. Bartolucci AA, Tendera M, Howard G. Meta-analysis of multiple primary prevention trials of cardiovascular events using aspirin. Am J Cardiol. 2011; 107:1796–1801.

39. Reiner Z, Catapano AL, De Backer G, Graham I, Taskinen MR, Wiklund O, et al. ESC/EAS Guidelines for the management of dyslipidaemias: the Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and the European Atherosclerosis Society (EAS). Eur Heart J. 2011; 32:1769–1818.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download