This article has been
cited by other articles in ScienceCentral.
The sulfites are widely used as anti-browning agents and preservatives in food, cosmetics and medicine. The use and amount of sulfites in products have recently been regulated by the Food and Drug Administration (FDA) and the Korean FDA; however, sulfite hypersensitivity reactions, ranging from bronchoconstriction,
1 urticaria,
2 contact dermatitis
3 to life threatening anaphylaxis,
4 are still being reported.
5 To date, this is the first study to compare two major phenotypes of sulfite hypersensitivity, asthma and urticaria, in this country. Moreover, we carefully analyzed the clinical features of sulfite sensitive asthma, and compared them with those of sulfite tolerant asthma.
We retrospectively analyzed the medical records of 26 sulfite hypersensitivity subjects confirmed by a sulfite oral provocation test (OPT). As a control group, 61 asthmatic patients negative to sulfite OPT were enrolled from Ajou University Hospital, Suwon, Korea. We performed sulfite oral provocation test on the subjects who were resistant to conventional treatment of asthma/urticaria or who had histories of allergy to sulfite containing food such as wine, dried fruits, or salads from buffet. To confirm sulfite sensitivity, we conducted single blind placebo controlled provocation test. After the asthmatic patients showed no response to gelatin capsule (EMBO CAPS lot. 051A51-22306, Suheung Capsule, Seoul, Korea) with 2 hours observation, they were administered sodium metabisulfite (Na
2S
2O
5; Sigma Co., St. Louis, MO, USA) starting from 40 mg and increasing to 100-200 mg via gelatin capsule vehicle. The reactions were observed for 2 hours each until the hypersensitivity reactions developed,
1 maximally 6 hours. Especially for bronchoconstriction reaction, 1) a decline in FEV
1 of 20% or greater, or 2) a decline in FEV
1 of 15% to 20% compared to the baseline with definite symptoms/signs of sulfite hypersensitivities (such as wheezing, cough or dyspnea) were considered positive. Pulmonary function tests were repeated at 30 minute intervals.
6 In the case of urticarial reaction, sulfite OPT was defined as positive when hives arise; however, it was classified as negative if subjective pruritus developed. Subjects were classified into group I: sulfite sensitive asthma, group II: sulfite sensitive urticaria and group III: sulfite tolerant asthma according to their sulfite OPT reaction. In addition, group I included two patients with concurrent sulfite anaphylaxis.
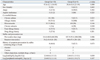
Sulfite sensitive asthma was a more common phenotype of sulfite hypersensitivity than sulfite sensitive urticaria (69.2% vs. 30.8%). Chronic asthma was more common in group I, as previously reported;
6 however, chronic urticaria was more common in group II. Group II subjects required significantly higher provocative dosage of sulfite (187.5±35.4 mg vs. 114.4±60.0 mg,
p=0.006) and a longer time (100±37.4 min vs. 54.2±34.8 min,
p=0.008) to induce hypersensitivity reactions compared with group I subjects. A previous history of adverse reactions was found in 44.4% of group I and 62.5% of group II, being different from previous studies that enrolled subjects with a history of sulfite hypersensitivities (
Table 1).
1,
7
There were no significant differences in asthma-related parameters-such as atopic status, total IgE level, peripheral eosinophil count, FEV
1% and metacholine PC
20 level when the clinical characteristics were compared between group I and group III. The prevalence of severe asthma according to American Thoracic Society guidelines was significantly higher in group I than as previously reported in group III (44% vs. 16%,
p=0.023).
1,
8 The asthma exacerbation related hospitalization rate (≥1 times/yr), emergency room visit (≥1 times/yr) and oral steroid burst (≥3 times/yr) were significantly higher in group I than in group III (66.7% vs. 24.6%,
p=0.001; 66.7% vs. 24.6%,
p=0.001; 55.6% vs. 17.3%,
p=0.003).
Many potential mechanisms of sulfite hypersensitivity have been postulated. Sulfite oxidase deficiency has been suggested as one of the mechanisms.
5 Most asthmatic patients could show a sensitivity to SO2 exposure due to bronchial hypersensitivity.
9 However, not all asthmatic patients with severe clinical symptoms presented sulfite hypersensitivity in this study, due to their higher prevalence of severe asthma and more frequent episodes of acute exacerbation compared to sulfite tolerant asthma. We speculate that these findings are due to individual variability of sulfite oxidase inactivity.
1 It would, therefore, be helpful to investigate individual factors, including genetic factors, in order to better understand the mechanism of sulfite hypersensitivity.
We report herein two major phenotypes of sulfite hypersensitivity, asthma and urticaria in Korea. Considering high prevalence of severe asthma and frequent health care utilization rate in patients with sulfite sensitive asthma, sulfite OPT can be considered as a screening test to identify exacerbating factors for severe asthma patients regardless of history of hypersensitivity reaction.
ACKNOWLEDGEMENTS
This study was supported by a grant from the Korean Health 21 R&D Project, the Ministry of Health & Welfare, Republic of Korea (A111218-11-PG01).
References
1. Cho YS, Baik SH, Park HS, Rhu NS, Cho DI, Kim JW. Clinical features of sulfite-sensitive asthmatics. Tuberc Respir Dis. 1992; 39:159–166.

2. Di Lorenzo G, Pacor ML, Mansueto P, Martinelli N, Esposito-Pellitteri M, Lo Bianco C, et al. Food-additive-induced urticaria: a survey of 838 patients with recurrent chronic idiopathic urticaria. Int Arch Allergy Immunol. 2005; 138:235–242.

3. García-Gavín J, Parente J, Goossens A. Allergic contact dermatitis caused by sodium metabisulfite: a challenging allergen: a case series and literature review. Contact Dermatitis. 2012; 67:260–269.

4. Schwartz HJ. Sensitivity to ingested metabisulfite: variations in clinical presentation. J Allergy Clin Immunol. 1983; 71:487–489.

5. Vally H, Misso NL, Madan V. Clinical effects of sulphite additives. Clin Exp Allergy. 2009; 39:1643–1651.

6. Gunnison AF, Jacobsen DW. Sulfite hypersensitivity. A critical review. CRC Crit Rev Toxicol. 1987; 17:185–214.

7. Stevenson DD, Simon RA. Sensitivity to ingested metabisulfites in asthmatic subjects. J Allergy Clin Immunol. 1981; 68:26–32.

8. Bush RK, Taylor SL, Holden K, Nordlee JA, Busse WW. Prevalence of sensitivity to sulfiting agents in asthmatic patients. Am J Med. 1986; 81:816–820.

9. Kim YY. Past, present, and future of allergy in Korea. Allergy Asthma Immunol Res. 2010; 2:155–164.








 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download