Abstract
Purpose
Although the analgesic effects of corticosteroids have been well documented, little information is available on periarticular injection (PI) containing corticosteroids for early postoperative pain management after total knee arthroplasty (TKA). We performed a prospective double-blind randomized trial to evaluate the efficacy and safety of an intraoperative corticosteroid PI in patients undergoing TKA.
Materials and Methods
Seventy-six consecutive female patients undergoing bilateral staged TKA were randomized to receive steroid or non-steroid PI, with 3 months separating the procedures. The steroid group received PI with a mixture containing triamcinolone acetonide (40 mg). The non-steroid group received the same injection mixture without corticosteroid. During the postoperative period, nighttime pain, functional recovery [straight leg raising (SLR) ability and maximal flexion], patient satisfaction, and complications were recorded. Short-term postoperative clinical scores and patient satisfaction were evaluated at 6 months.
Results
The pain level was significantly lower in the PI steroid than the non-steroid group on the night of the operation (VAS, 1.2 vs. 2.3; p=0.021). Rebound pain was observed in both groups at POD1 (VAS, 3.2 vs. 3.8; p=0.248), but pain remained at a low level thereafter. No significant differences were seen in maximal flexion, frequency of acute rescuer, clinical scores, and patient satisfaction. The steroid group was able to perform SLR earlier than the non-steroid group (p=0.013). The incidence of complications was similar between the groups.
Total knee arthroplasty (TKA) has been reported to be the most effective procedure for pain relief in patients with advanced osteoarthritis. Ironically, however, many patients are concerned of immediate postoperative pain, preoperatively and actually experience considerable pain after surgery.1,2 Multimodal and pre-emptive approaches are currently used for perioperative pain relief. Periarticular injection (PI) of a drug cocktail has been particularly effective in decreasing pain and improving early function after TKA.3-11 However, there is no gold standard protocol regarding the drug composition and amount for the cocktail injection.
The mainstays of multimodal therapeutic approaches, including drug cocktails, have been opioids.8,12 However, the effectiveness of opioids is limited by side effects such as respiratory depression, urinary retention, nausea, and vomiting.12 The use of systemic corticosteroids in a multimodal analgesic protocol is documented to be efficacious in the treatment of postoperative pain, nausea, and vomiting.12-14 In contrast, there is little information on the role of corticosteroid in PI treatment for early postoperative pain management. Previous studies that used a PI drug cocktail, with or without a corticosteroid, have documented pain relief and functional recovery, but did not examine the effect of the corticosteroid.3-5,7-11
Therefore, we performed a prospective, randomized, double-blind study to determine 1) whether a PI containing a corticosteroid provides an additional pain relief and functional recovery effect and; 2) whether a PI containing a corticosteroid increases perioperative complications after TKA.
Ninety female patients scheduled for bilateral TKAs provided informed consent to participate in this prospective, double-blinded study conducted from March 2010 to December 2011. Eligible patients included 90 female patients between the ages of 50 and 90 years who were scheduled to undergo staged bilateral TKA (3-month interval) for the treatment of primary advanced osteoarthritis. Of the 90 eligible patients, 8 were not enrolled because of refusal to participate (n=5), major depressive disorder (n=1), and other diagnostic reasons (n=2) (Fig. 1). Thus, 82 patients were recruited, and one knee was randomly assigned to the steroid or non-steroid PI group. After 3 months, the contralateral knee was assigned to the opposite group. The steroid group received a PI containing corticosteroid (40 mg of triamcinolone acetonide), and the non-steroid group received a PI without corticosteroid. Randomization was performed using a computer-generated permutation table of 4 and 6 blocks. Six of the 82 patients were excluded because of transfer due to congestive heart failure (n=1), transfer due to gastrointestinal bleeding (n=1), delirium after surgery (n=1), and failed spinal anesthesia (n=3). Thus, 76 female patients were included in the final analysis (Fig. 1). The patients and an independent investigator who prospectively collected the clinical information were unaware of the group assignments until the final data analysis was completed. There were all female patients with 69.3 years (mean age, ranging from 62 to 77). The median body mass index was 25.9 kg/m2 (range, 22-32). The study protocol was approved by our Institutional Review Board and registered at Clinical Trials.
All patients received the same spinal anesthetic and multimodal protocol to improve pain relief. Oral analgesic pills (200 mg celecoxib, 362.5 mg ultracet, and 75 mg pregabalin) were administered for preemptive analgesia on the morning of the operation. Spinal anesthesia, using 0.5% bupivacaine, was administered by an anesthesiologist; throughout surgery, anesthesia was maintained with propofol using a target-controlled device.
All surgeries were performed before noon by a single surgeon (S.K.K) using a standard medial parapatellar arthrotomy with a tourniquet. A posteriorly stabilized prosthesis (PFC sigma; DePuy, Johnson & Johnson, Warsaw, USA) was implanted in all patients. The patella was unresurfaced in all cases, and cement fixation was used for all components.
A multimodal PI cocktail (100 mL) consisting of 10 mg morphine sulfate, 300 mg ropivacaine, 30 mg ketorolac, 300 µg of 1:1000 epinephrine, normal saline, and with or without a corticosteroid (40 mg of triamcinolone acetonide) was prepared in two 50-mL syringes. Before the implants were fixed with cement, 50 mL of the cocktail were injected into the posterior capsule, sheath of the medial and lateral collateral ligaments, and soft tissue above the medial and lateral femoral epicondyle. After insertion of polyethylene bearings, a second 50 mL were injected into the peri-pes anserinus, capsule, quadriceps, subcutaneous tissue, and peri-patella aponeurosis.
All patients received postoperative intravenous patient-controlled analgesia (PCA), which was programmed to provide a continuous infusion of 500 µg fentanyl and 30 mL of 0.5% bupivacaine, at a rate of 4 mL/h, with an on-demand bolus infusion of 2 mL with a 10-min lockout period. When patients resumed oral analgesic intake, 200 mg celecoxib and 362.5 mg ultracet were administered every 12 hours. An intramuscular injection of ketoprofen (100 mg) was used as an acute rescuer when a patient reported severe pain greater than 7 on a 0 to 10 Visual Analog Scale (VAS). The continuous intravenous PCA was typically discontinued on the third postoperative day. The patient knees were placed daily in a continuous-passive-motion machine starting at 2 days after the operation until discharge. All patients were discharged on postoperative day 9.
A clinical investigator who was blinded to the group assignments reviewed the diagnosis and medical histories, and prospectively collected the demographic data. Preoperative clinical status was evaluated using the knee motion arc, American Knee Society (AKS) knee and function scores, and Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) scores. Postoperative clinical evaluation was performed at 6 months using the same scales. Flexion contracture and maximum flexion were measured to the nearest 5° by an investigator using a standard clinical goniometer with the patients in a supine position.
The primary outcome variable was the postoperative nighttime pain levels on the night of the surgery and at postoperative days 1, 2, 3, and 7. The pain levels were recorded by a clinical investigator using a VAS that ranged from 0 (no pain) to 10 (worst imaginable pain) at each time point. The secondary outcome variables were functional recovery [ability for straight leg raising (SLR) and maximal flexion], patient satisfaction, and complications. The level of patient satisfaction was obtained using a VAS on the operation night and postoperatively on days 1 and 7, as well as at 6 months. Wound complications, including periprosthetic joint infection, wound oozing, and wound dehiscence, were evaluated. In addition, patients were observed for narcotic-related side effects, including nausea, vomiting, respiratory depression, and urinary retention.
Statistical analyses were conducted using SPSS for Windows Version 15 (SPSS Inc., Chicago, IL, USA). Chi-squared test or Fisher's exact test was used to determine the differences in categorical variables (frequency of acute rescuer and incidence of wound complications). Continuous variables (pain, functional recovery, clinical score, and patient satisfaction) were analyzed using Student's t-test. An a priori power analysis using a 2-sided hypothesis test, at an alpha level of 0.05 and a power of 80%, was used to determine the sample size statistical power. One hundred-twenty knees were required to detect a difference of 1 in the VAS pain level, which we considered as clinically significant. Accordingly, the study sample size (152 knees) had sufficient power to detect a difference.
The pain level was significantly lower in steroid-treated knees compared to non-steroid treated knees on the night of the operation (VAS, 1.2 vs. 2.3; p=0.021). Interestingly, rebound pain was observed in both groups 1 day after the surgery (VAS, 3.2 vs. 3.8; p=0.248). Thereafter, pain remained at a low level and was not different between the 2 groups. In addition, the level of patient satisfaction decreased from the night of operation to the night of POD1 in both the steroid PI (VAS, 9.0 to 7.8) and non-steroid PI groups (VAS, 8.8 to 8.0) (Fig. 2).
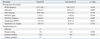
There were no significant differences in the secondary variables, including hemovac drainage, maximal flexion (postoperative day 7), and frequency of acute rescuer (postoperative day 2). The steroid PI group was able to perform SLR earlier than the non-steroid PI group (p=0.013) (Table 1). The 2 groups demonstrated similar postoperative range of motion, AKS score, WOMAC score, and patient satisfaction at 6 months after TKA.
The addition of corticosteroid did not increase the risk for wound complications, including infection or narcotic-related side effects, during the immediate postoperative period (Table 2).
The analgesic effects of corticosteroids have been well documented.12,14,15 However, little information is available on the role of corticosteroid PI in providing immediate postoperative pain relief and functional recovery after TKA. The most important finding of the current prospective study is that a PI containing a corticosteroid provided an additional pain-relieving effect on the night of the operation but did not influence pain levels for the remainder of the postoperative period. This study also confirms the safety of a PI containing a corticosteroid for use in a multimodal analgesic protocol.
In previous studies of PI, some PI cocktails contained a corticosteroid whereas others did not. Irrespective of corticosteroid use, studies have documented an improvement in pain relief and functional recovery with PI, compared to a non-PI group.3-9 The current findings support these earlier observations by demonstrating that a PI with a corticosteroid provides additional pain relief on the night of the operation. However, our findings are in contrast to those of another study that compared PIs with and without a corticosteroid and found no effect of the corticosteroid on pain relief and functional recovery.10 Importantly, the above study assessed patient pain levels only 1 day after surgery and on the day of discharge. We found that an additional pain-relieving effect of corticosteroid PI appeared only on the night of the operation; therefore, this previous work might have failed to examine the critical period. In addition to a beneficial effect early after surgery, PI with triamcinolone also appeared to have a improved pain-relieving effect on postoperative days 1-7, as the steroid PI group demonstrated a lower, albeit non-significant, overall pain level during this period compared to the non-steroid PI group (Fig. 2). The half-life of triamcinolone ranges from 18 to 36 hours, and intramuscular administration of this acetate form provides slow absorption but a prolonged duration.12 Patient pain is maximized at night because of minimal environmental stimuli, therefore, we focused on nighttime pain for the duration of our study. Overall, our findings are noteworthy, considering a multimodal PI protocol for postoperatively practical pain management.
One interesting finding was that rebound pain was observed in both groups 1 day after surgery (night-time), and that the increase in pain was higher in the steroid PI group (+2.0) than in the non-steroid group (+1.5). When comparing our study with previous PI works, we found that rebound pain level (amount of change) was the highest on 1 day after surgery (Table 3). In the studies of Lombardi, et al.5 and Vendittoli, et al.9 there was no rebound pain on POD1. Their cocktail components were different from those used by recent studies, which included no morphine and low-dose local anesthetic. Recent studies of Busch, et al.,3 Koh, et al.,4 and Mullaji, et al.6 observed rebound pain on POD1. Their cocktail components including drug composition and amount were similar to ours. Although the VAS pain scores increased by 1-2 point on POD1, we are not certain of it's clinical significance. Importantly, rebound pain might directly influence patient satisfaction negatively (Fig. 1). Thus, if a multimodal analgesic protocol cannot decrease rebound pain, an additional pain-relieving effect of corticosteroid on the night of the operation may be meaningless.
This study also suggests that a PI with corticosteroid partially improves functional recovery. The steroid PI group was able to perform SLR earlier than the non-steroid PI group, but maximal flexion was same between groups. This finding concurs with that of a PI study by Koh, et al.,4 but most previous studies have reported that PI does not improve functional recovery.3,5,7-10
Several studies have reported that intra-articular corticosteroid injection might increase the risk of joint complications.16,17 However, Sauerland, et al.15 found no adverse effects in patients who were treated perioperatively with methylprednisolone during major surgery. In line with this, several other studies focused on PI also reported no increase in the risk of postoperative complications after corticosteroid PI.7,8,10 Our study also supports the notion that corticosteroid PI does not increase perioperative complications after TKA. There were no incidences of infection; minimal wound oozing was observed but was not different between groups.
Our study has several limitations. First, this study assessed bilateral staged TKA, with the surgeries separated by 3 months. According to the gate-control theory,18 if one side has a severe pain level after surgery, the other side might feel a reduced pain level in bilateral simultaneous TKA. Thus, our study conducted bilateral staged surgery instead of bilateral simultaneous surgery. However, a distorted pain level may be experienced after the second operation (3 months later) due to recall of previous pain from the first operation. Second, our study participants were all Korean women, therefore, our findings may not be generalized to other patient populations other than elderly females (mean age, 69.3 years). However, since culture, gender, and age can influence the subjective perception of pain, we believe that our population findings are relevant. Finally, a single clinical investigator assessed only nighttime pain level, which is thought to be a good method to use in pain studies. Even though all surgeries were conducted before noon, the timing of pain assessment after surgery differed between patients. Furthermore, some patients were asked to recall pain levels; therefore, recall bias and observer bias may be involved.
In conclusion, PI containing a corticosteroid provided an additional pain-relieving effect on the night of the operation, but not for the remainder of the postoperative period. Because of the rebound in the level of pain at postoperative day 1, the lower pain immediately after the surgery with steroid treatment does not guarantee sustained pain relief after TKA. Further studies focused on sustaining a low level of pain level, without rebound, during the postoperative period should be conducted. In addition, corticosteroid PI did not increase perioperative complications after TKA, which confirms the safety of PI containing corticosteroid for use in a multi-modal analgesic protocol.
Figures and Tables
 | Fig. 1Subject screening flowchart. PI, periarticular injection; CHF, congestive heart failure; GI, gastrointestinal; TKA, total knee arthroplasty. |
 | Fig. 2Summary of nighttime pain levels (bar graph) and patient satisfaction (line graph). OP night, operation night; POD1, 2, 3, and 7; 1, 2, 3, and 7 days postoperatively. *Indicates significance (p<0.05; 95% confidence intervals). VAS, Visual Analog Scale. |
References
1. Park KK, Shin KS, Chang CB, Kim SJ, Kim TK. Functional disabilities and issues of concern in female Asian patients before TKA. Clin Orthop Relat Res. 2007; 461:143–152.

2. Trousdale RT, McGrory BJ, Berry DJ, Becker MW, Harmsen WS. Patients' concerns prior to undergoing total hip and total knee arthroplasty. Mayo Clin Proc. 1999; 74:978–982.

3. Busch CA, Shore BJ, Bhandari R, Ganapathy S, MacDonald SJ, Bourne RB, et al. Efficacy of periarticular multimodal drug injection in total knee arthroplasty. A randomized trial. J Bone Joint Surg Am. 2006; 88:959–963.

4. Koh IJ, Kang YG, Chang CB, Kwon SK, Seo ES, Seong SC, et al. Additional pain relieving effect of intraoperative periarticular injections after simultaneous bilateral TKA: a randomized, controlled study. Knee Surg Sports Traumatol Arthrosc. 2010; 18:916–922.

5. Lombardi AV Jr, Berend KR, Mallory TH, Dodds KL, Adams JB. Soft tissue and intra-articular injection of bupivacaine, epinephrine, and morphine has a beneficial effect after total knee arthroplasty. Clin Orthop Relat Res. 2004; 125–130.

6. Mullaji A, Kanna R, Shetty GM, Chavda V, Singh DP. Efficacy of periarticular injection of bupivacaine, fentanyl, and methylprednisolone in total knee arthroplasty: a prospective, randomized trial. J Arthroplasty. 2010; 25:851–857.

7. Parvataneni HK, Shah VP, Howard H, Cole N, Ranawat AS, Ranawat CS. Controlling pain after total hip and knee arthroplasty using a multimodal protocol with local periarticular injections: a prospective randomized study. J Arthroplasty. 2007; 22:6 Suppl 2. 33–38.

8. Ranawat AS, Ranawat CS. Pain management and accelerated rehabilitation for total hip and total knee arthroplasty. J Arthroplasty. 2007; 22:7 Suppl 3. 12–15.

9. Vendittoli PA, Makinen P, Drolet P, Lavigne M, Fallaha M, Guertin MC, et al. A multimodal analgesia protocol for total knee arthroplasty. A randomized, controlled study. J Bone Joint Surg Am. 2006; 88:282–289.
10. Christensen CP, Jacobs CA, Jennings HR. Effect of periarticular corticosteroid injections during total knee arthroplasty. A double-blind randomized trial. J Bone Joint Surg Am. 2009; 91:2550–2555.

11. Han CD, Lee DH, Yang IH. Intra-synovial ropivacaine and morphine for pain relief after total knee arthroplasty: a prospective, randomized, double blind study. Yonsei Med J. 2007; 48:295–300.

12. Salerno A, Hermann R. Efficacy and safety of steroid use for postoperative pain relief. Update and review of the medical literature. J Bone Joint Surg Am. 2006; 88:1361–1372.
13. Gilron I. Corticosteroids in postoperative pain management: future research directions for a multifaceted therapy. Acta Anaesthesiol Scand. 2004; 48:1221–1222.

14. Wang JJ, Ho ST, Lee SC, Tang JJ, Liaw WJ. Intraarticular triamcinolone acetonide for pain control after arthroscopic knee surgery. Anesth Analg. 1998; 87:1113–1116.

15. Sauerland S, Nagelschmidt M, Mallmann P, Neugebauer EA. Risks and benefits of preoperative high dose methylprednisolone in surgical patients: a systematic review. Drug Saf. 2000; 23:449–461.

16. Armstrong RW, Bolding F, Joseph R. Septic arthritis following arthroscopy: clinical syndromes and analysis of risk factors. Arthroscopy. 1992; 8:213–223.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download