Abstract
Purpose
This study was to evaluate the relationship of 25(OH)D3 levels with anticonvulsant use and other possible factors in epileptic children and adolescents.
Materials and Methods
We studied 143 patients with epilepsy (90 boys, 53 girls; 11.21±4.49 years), who had been treated with anticonvulsants for more than 1 year. Patients who had taken multiple vitamins before the blood test and those who have the limitation of physical activity (wheelchair-bound) were excluded from the study. We evaluated the difference in vitamin D status according to the type and number of anticonvulsants taken and other factors such as gender, age, intelligence and seizure variables.
Results
For patients with mental retardation or developmental delay, 25(OH)D3 levels were lower than the levels in patients with normal intelligence quotient levels (p=0.03). 25(OH)D3 levels were lower in patients who had taken anticonvulsants for more than 2 years as compared to those who had taken them for less than 2 years (p=0.03). Those taking oxcarbazepine had significantly lower vitamin D levels than patients taking valproic acid (p=0.01). However, no effects of number of anticonvulsants taken were detectable. More than two-thirds of the patients were diagnosed with osteopenia or osteoporosis in patients showing either vitamin D insufficiency or deficiency.
Vitamin D is an essential nutrient that maintains the homeostasis of calcium and phosphorous levels in the body. The importance of vitamin D was recently emphasized when it was reported to be involved in cell differentiation and proliferation, immune function, and the prevention and treatment of certain cancers.1,2 Research has shown that adult epilepsy patients can exhibit a deficiency of vitamin D.3,4 In particular, hepatic enzyme-inducing antiepileptic drugs, such as phenytoin and carbamazepine, induce cytochrome P450 enzymes in the liver, thereby promoting the catabolism of vitamin D and its derivatives. This lowers vitamin D levels, resulting in the hypocalcemia and secondary hyperparathyroidism that eventually leads to a loss in bone mineral density (BMD).5-7 In pediatric patients, however, controversies still remain regarding the effect of anticonvulsants on vitamin D levels and bone health.8
The objective of this study was to evaluate vitamin D status in epileptic children and adolescents taking anticonvulsants and to analyze the associations of various factors with vitamin D levels, including the type and number of anticonvulsants taken, seizure type, and patient age at seizure onset, as well as patient age, gender, and intelligence and developmental status.
We performed a retrospective review of the medical records of epileptic children and adolescents who visited the pediatric epilepsy clinic at Korea University Guro Hospital between June and November 2012. Patients who had taken anticonvulsants for more than 1 year were included. To minimize the effects of confounding factors, patients who had taken multiple vitamins before the blood test and those who have the limitation of physical activity (wheelchair-bound) were excluded from the study.
Levels of calcium, phosphorus, magnesium, ionized calcium, parathyroid hormone, 25(OH)D3, 1,25(OH)2D3, and alkaline phosphatase, as well as the date of blood sampling were recorded. In addition, patient age and gender, underlying disease, brain magnetic resonance imaging (MRI) findings, intelligence and developmental status, patient age at seizure onset, type of seizure, number of seizures over the past 3 months ("well controlled," if seizure free), type of anticonvulsant, and duration of anticonvulsant use were also elucidated from the medical records. Intelligence and developmental status was evaluated using the Korean Bayley Scale of Infant Development-II (K-BSID-II), Korean Wechsler Preschool and Primary Scale for Intelligence (K-WPPSI), and Korean Wechsler Intelligence Scale for Children, 3rd edition (K-WISC-III), according to the age of the patient at the initial epilepsy evaluation. In cases where K-BSID-II score signified developmental delay, where the intelligence quotient (IQ) was <70 according to the results of K-WPPSI or K-WISC-III, or where the patient was attending a special class, the patient was diagnosed with mental retardation or developmental delay.9
The concentration of 25(OH)D3 was considered normal when >30 ng/mL, insufficient when 20-30 ng/mL, and deficient when <20 ng/mL.10,11
Bone density was measured in patients whose 25(OH)D3 concentration was <30 ng/mL using a Hologic dual-energy X-ray absorptiometry system (Discovery QDR 4500W, Hologic, Waltham, MA, USA). According to the WHO criteria, normal bone density was considered when the Z score was >-1.0, osteopenia was considered when the Z score was between -2.5 and -1.0, and osteoporosis was considered when the Z score was <-2.5.12
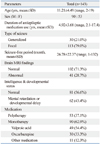
In this study of 143 pediatric patients, there were 90 boys and 53 girls. The average age at diagnosis of epilepsy was 6.25±4.24 years (range, 2-17 years) and the average duration of anticonvulsant use was 4.92±3.68 years (range, 1.1-17.4 years). Among the study participants, 41 patients (28.7%) had brain lesions on MRI, and 102 patients (71.3%) had normal brain MRI findings. The numbers of patients with focal and generalized epilepsy were 113 (79.0%) and 30 (21.0%), respectively. IQ and developmental tests showed that 81 (56.6%) of the patients were of normal intelligence, and 62 (43.4%) had mental retardation or developmental delay. The minority of patients were receiving polydrug therapy (53; 37.1%), and 90 patients (62.9%) were receiving monodrug therapy: valproic acid (n=49), oxcarbazepine (n=30), lamotrigine (n=4), phenobarbital (n=2), levetiracetam (n=2), zonisamide (n=1), carbamazepine (n=1), and topiramate (n=1) (Table 1).
In the present study, 25(OH)D3 levels were measured in the summer and fall seasons (between June and November). The average 25(OH)D3 level was 34.26±10.93 ng/mL (range, 12.1-59.1 ng/mL) (Table 2). We identified that patients' vitamin D levels followed a normal distribution (p=0.2) by conducting t-test and linear regression (data not shown). Thirteen patients (13/143; 9.1%) had vitamin D deficiency with 25(OH)D3 levels <20 ng/mL, 40 (40/143; 28.0%) had a vitamin D insufficiency with levels between 20 and 30 ng/mL, and 90 (90/143; 62.9%) had normal vitamin D status with levels >30 ng/mL.
There was no difference in 25(OH)D3 levels between boys and girls (34.33±10.80 ng/mL vs. 34.13±11.24 ng/mL; p=0.92), patients with generalized epilepsy and focal epilepsy (32.26±11.50 ng/mL vs. 35.06±10.64 ng/mL; p=0.17), patients with well and poorly controlled epilepsy (34.23±11.50 ng/mL vs. 34.47±9.17 ng/mL; p=0.82), or patients with normal and abnormal brain MRI findings (34.10±10.53 ng/mL vs. 34.85±12.39 ng/mL; p=0.74).
A negative correlation was noted between age and 25(OH)D3 levels by linear regression. As the age of patients increased, 25(OH)D3 levels decreased (p<0.05). In patients with mental retardation or developmental delay, 25(OH)D3 levels were significantly lower than those in patients with normal IQ (31.99±10.59 ng/mL vs. 35.99±10.93 ng/mL; p=0.03). The duration of anticonvulsant use also influenced 25(OH)D3 levels; patients who had taken anticonvulsants for more than 2 years had significantly lower levels of 25(OH)D3 than those who had taken these medications for less than 2 years (32.87±11.00 ng/mL vs. 37.08±10.40 ng/mL; p=0.03). There was no difference in vitamin D levels between patients receiving mono- and polytherapy (33.79±10.93 ng/mL vs. 35.04±11.00 ng/mL; p=0.51). However, among patients receiving monotherapy, those taking oxcarbazepine, a hepatic enzyme inducer, had significantly lower vitamin D levels than patients taking valproic acid, a hepatic enzyme inhibitor (30.41±9.53 ng/mL vs. 36.83±11.66 ng/mL; p=0.01) (Table 3).
Of the 13 patients (9.1%) with vitamin D insufficiency and 40 patients (28.0%) with vitamin D deficiency, 32 patients (32/53; 60.4%) underwent bone mineral density measurements in the hip and lumbar spine; their Z scores were distributed between -4 and 1.3. In total, 9 patients (9/32; 28.1%) exhibited osteoporosis (Z≤-2.5), 13 patients (13/32; 40.6%) exhibited osteopenia (-2.5<Z<-1.0), and 10 patients (10/32; 31.3%) had normal bone density (Z≥-1.0). Among the group with vitamin D insufficiency, 5 patients (5/21; 23.8%) were diagnosed with osteoporosis, 9 patients (9/21; 42.9%) with osteopenia, and 7 patients (7/21; 33.3%) were normal. In addition, among the group with vitamin D deficiency, 4 patients (4/11; 36.4%) had osteoporosis, 4 (4/11; 36.4%) had osteopenia, and 3 (3/11; 27.2%) were normal.
The objective of this study was to investigate the associations between vitamin D levels and various factors related with pediatric epilepsy, including type and number of anticonvulsants and seizure variables, as well as patient gender, age, and intelligence and developmental status.
We noted that there is no difference in 25(OH)D3 levels according to gender, seizure type, presence of brain lesion on MRI, and the number of anticonvulsant medications used.
25(OH)D3 levels were found to decline as age increased. A previous study examining adults between the ages of 20 and 96 years indicated that 25(OH)D3 concentration decreases as age increases. This might be related to age-related biological processes or the fact that social and physical activities become limited as people age.13 In addition, a research on 25(OH)D3 deficiency over children and adolescents shows the same result that prevalence of vitamin D deficiency increases with age.14 Concerning this result, there is a possibility of less sunlight exposure for older children because they may stay indoors longer during the day to study. Yet, further studies about age-related biological process in pediatric age are warranted to clarify this relationship.
Patients on polytherapy demonstrated significantly lower 25(OH)D3 levels than patients on monotherapy in a previous study.15 However, we noted no difference in 25(OH)D3 levels between patients receiving mono- and polytherapy.
It has been reported that the effect of type and number of anticonvulsants on 25(OH)D3 levels.16 There have been several studies that patients taking oxcarbazepine (hepatic enzyme inducing drug) have significantly lower 25(OH)D3 levels.7,17 In order to investigate if a difference between hepatic enzyme inducing drug and non-hepatic enzyme inducing drug exists, a comparison between oxcarbazepine and valproic acid was made. Our study demonstrated the same results.
The duration of anticonvulsant use was associated with 25(OH)D3 levels, which were significantly lower in patients who had taken anticonvulsants for more than 2 years than in patients who had taken them for less than 2 years. One previous study showed that patients who took anticonvulsants for more than 2 years had normal vitamin 25(OH)D3 D levels, although BMD was found to be decreased; these authors mentioned the possibility of high vitamin D intake among the experimental group.18 However, we ruled out any vitamin supplementation in our study.
The developmental status and IQ level were also associated with 25(OH)D3 levels. 25(OH)D3 levels among patients with mental retardation or developmental delay were lower than those in patients with normal IQs, a finding that has been reported previously.19 Because social and outdoor activities may be limited for these patients, 25(OH)D3 levels may consequently be influenced.
Finally, more than two-thirds of the patients (72.8%) were diagnosed with osteopenia or osteoporosis in patients showing vitamin D deficiency. Even in patients showing vitamin D insufficiency, 66.7% of the patients were diagnosed with osteopenia or osteoporosis.
The main limitation of this study was that 25(OH)D3 levels were not measured before anticonvulsant use. In addition, we followed the WHO criteria in evaluating bone density because there was no reference range for Korean children and adolescents.
In conclusion, the possibility of vitamin D deficiency in children and adolescents taking anticonvulsants should be considered in daily practice. This is especially important in pediatric patients with mental retardation or developmental delay and patients who have been taking anticonvulsants for more than 2 years or taking hepatic enzyme inducing drugs.
ACKNOWLEDGEMENTS
This work was supported by a grant from the fund, Korea University College of Medicine.
References
1. Misra M, Pacaud D, Petryk A, Collett-Solberg PF, Kappy M. Drug and Therapeutics Committee of the Lawson Wilkins Pediatric Endocrine Society. Vitamin D deficiency in children and its management: review of current knowledge and recommendations. Pediatrics. 2008; 122:398–417.

2. Rovner AJ, O'Brien KO. Hypovitaminosis D among healthy children in the United States: a review of the current evidence. Arch Pediatr Adolesc Med. 2008; 162:513–519.

4. Pack A. Bone health in people with epilepsy: is it impaired and what are the risk factors? Seizure. 2008; 17:181–186.

5. Pack AM, Morrell MJ. Adverse effects of antiepileptic drugs on bone structure: epidemiology, mechanisms and therapeutic implications. CNS Drugs. 2001; 15:633–642.

6. Bell RD, Pak CY, Zerwekh J, Barilla DE, Vasko M. Effect of phenytoin on bone and vitamin D metabolism. Ann Neurol. 1979; 5:374–378.

7. Mintzer S, Boppana P, Toguri J, DeSantis A. Vitamin D levels and bone turnover in epilepsy patients taking carbamazepine or oxcarbazepin. Epilepsia. 2006; 47:510–515.

8. Shellhaas RA, Joshi SM. Vitamin D and bone health among children with epilepsy. Pediatr Neurol. 2010; 42:385–393.

9. Kwon J. Diagnostic evaluation and rehabilitation in children with intellectual disabilitie. J Korean Med Assoc. 2009; 52:601–610.

10. Bischoff-Ferrari HA, Giovannucci E, Willett WC, Dietrich T, Dawson-Hughes B. Estimation of optimal serum concentrations of 25-hydroxyvitamin D for multiple health outcomes. Am J Clin Nutr. 2006; 84:18–28.

11. Grant WB, Holick MF. Benefits and requirements of vitamin D for optimal health: a review. Altern Med Rev. 2005; 10:94–111.
12. Kanis JA. WHO Study Group. Assessment of fracture risk and its application to screening for postmenopausal osteoporosis: synopsis of a WHO report. Osteoporos Int. 1994; 4:368–381.

13. Baker MR, Peacock M, Nordin BE. The decline in vitamin D status with age. Age Ageing. 1980; 9:249–252.

14. Kumar J, Muntner P, Kaskel FJ, Hailpern SM, Melamed ML. Prevalence and associations of 25-hydroxyvitamin D deficiency in US children: NHANES 2001-2004. Pediatrics. 2009; 124:e362–e370.

15. Nettekoven S, Ströhle A, Trunz B, Wolters M, Hoffmann S, Horn R, et al. Effects of antiepileptic drug therapy on vitamin D status and biochemical markers of bone turnover in children with epilepsy. Eur J Pediatr. 2008; 167:1369–1377.

16. Shellhaas RA, Joshi SM. Vitamin D and bone health among children with epilepsy. Pediatr Neurol. 2010; 42:385–393.

17. Cansu A, Yesilkaya E, Serdaroğlu A, Hirfanoğlu TL, Camurdan O, Gülbahar O, et al. Evaluation of bone turnover in epileptic children using oxcarbazepine. Pediatr Neurol. 2008; 39:266–271.

18. Guo CY, Ronen GM, Atkinson SA. Long-term valproate and lamotrigine treatment may be a marker for reduced growth and bone mass in children with epilepsy. Epilepsia. 2001; 42:1141–1147.

19. Vanlint S, Nugent M, Durvasula S. Vitamin D and people with intellectual disability. Aust Fam Physician. 2008; 37:348–351.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download