Abstract
Purpose
This study was undertaken to assess the feasibility of early feeding in patients that have undergone emergency gastrointestinal (GI) surgery.
Materials and Methods
The authors retrospectively reviewed 84 patients that underwent emergency bowel resection and/or anastomosis from March 2008 to December 2011. Patients with severe shock, intestinal ischemia, sustained bowel perforation, or short bowel syndrome were excluded. Patients were divided into the early (group E; n=44) or late (group L; n=40) group according to the time of feeding commencement. Early feeding was defined as enteral feeding that started within 48 hours after surgery. Early and late feeding groups were compared with respect to clinical data and surgical outcomes.
Results
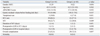
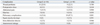
The most common cause of operation was bowel perforation, and the small bowel was the most commonly involved site. No significant intergroup differences were found for causes, sites, or types of operation. However, length of stay (LOS) in the intensive care unit (1 day vs. 2 days, p=0.038) and LOS in the hospital after surgery were significantly greater (9 days vs. 12 days, p=0.012) in group L than group E; pulmonary complications were also significantly more common (13.6% vs. 47.5%, p=0.001) in group L than group E.
Nutritional support plays important roles in wound healing and postoperative recovery,1,2 and a poor nutritional status is strongly associated with delayed wound healing and longer hospital stays after surgery.3,4 In particular, after emergency gastrointestinal (GI) surgery, nutritional status is impaired and basal energy expenditure is elevated,5,6 and thus, nutritional support is of considerable importance. Several reports have emphasized that early enteral feeding should be started as soon as possible after resuscitation because the immunomodulatory effect of enteral feeding could assist recovery.7-9 Furthermore, enhanced recovery after surgery has been shown to improve postoperative recovery after elective GI surgery.10,11 However, patients that undergo emergency GI surgery have an edematous or ischemic bowel, and are at high risk of postoperative complications, such as ileus, obstruction, or anastomotic failure. For these reasons, the majority of surgeons are wary of early feeding after emergency GI surgery. Furthermore, relatively few reports have been issued on the safety of early feeding after emergency GI surgery.6,12,13 Thus, this study was undertaken to assess the feasibility of early feeding in patients after emergency GI surgery.
We retrospectively reviewed the medical records of patients that underwent emergency GI surgery by a single surgeon from March 2008 to December 2011. The patients considered for inclusion in the present study had all undergone bowel resection and/or anastomosis. Patients that underwent simple appendectomy, cholecystectomy, primary repair of perforated viscera, or adhesiolysis without bowel anastomosis were excluded, as were patients with severe shock, sustained intestinal ischemia, uncontrolled bowel perforation, or short bowel syndrome. Additionally, to minimize severity differences in the study population, patients managed in the intensive care unit (ICU) for more than 3 days were also excluded.
Patients were allocated to an early group (E) or a late group (L) according to time of feeding commencement. Early feeding was defined as commencement of a liquid or soft diet via a tube or per os within 48 hours after surgery. The criteria to start enteral feeding included hemodynamic stability, secure bowel anastomosis performed, and no ischemic change of bowel observed in the operating room.
Feeding was started following the protocol described in Fig. 1. Tube feeding was selected when patient had a nasogastric, nasoenteric, or jejunostomy tube according to the patient's condition, such as mentality or underlying cerebral infarction, or a nasojejunal tube due to the gastric surgery. According to the recommendations of the Society of Parenteral and Enteral Nutrition, the purpose of early feeding is to promote early recovery and reduce time to discharge and postoperative complications. This study received Institutional Review Board approval.
Group clinical data and surgical outcomes were compared. The clinical data consisted of age, gender, Acute Physiology and Chronic Health Evaluation (APACHE) II score14 on ICU admission, causes of operation, operation type, operation sites, ratio of ICU care, vasopressor use and mechanical ventilation (MV), and the duration of MV. Surgical outcomes consisted of complication rates, complication types, and postoperative lengths of stay (LOS) in the hospital and in the ICU.
The criteria for ICU admission included advanced age (70≥age), presence of comorbidities, hemodynamic instability, and ventilator-dependent postoperative status, as well as patients that required intensive monitoring and may have potentially needed immediate intervention. Severe shock was defined as a mean arterial pressure maintained at >60 mm Hg (or >80 mm Hg if the patient had baseline hypertension) with the support of a vasopressor, such as high dose dopamine (>15 mcg/kg/min) or norepinephrine (>0.25 mcg/kg/min), after adequate fluid resuscitation.15 Short bowel syndrome was defined as <1.5 meters of small intestine remaining after surgery.16 Pulmonary complications include pneumonia, atelectasis, and pleural effusion. Diarrhea was defined as >3 times per day and/or a stool volume in excess of 500 mL/day. Ileus was defined as partial or complete non-mechanical blockage of the intestine as confirmed by simple abdominal radiography.
All values are presented as percentages or medians and ranges. Categorical variables were analyzed using the chi-square test, and continuous variables were analyzed using Student's t-test. Statistical analysis was performed using SPSS software version 18.0 (SPSS Inc., Chicago, IL, USA). Statistical significance was accepted for p values of <0.05.
One hundred and twelve patients were initially considered for this study. However, 9 patients were excluded based on a contraindication to enteral feeding (four cases of severe shock, three cases of short bowel syndrome, one intestinal ischemia, and one sustained bowel perforation), and another 19 were excluded due to ICU stay of more than 3 days after surgery. Finally, 84 patients were included the study cohort. There were 47 men and 37 women, and their median age was 64 years (range, 16-102). Median APACHE II score on ICU admission was 16 (range, 10-34). Fifty-three patients (63.1%) were managed in the ICU, and seven (8.3%) required a vasopressor due to immediate postoperative hypotension. Accompanying MV was performed in 22 patients (26.2%), and the median duration thereof was 1 day (range, 1-3). Median postoperative LOSs in the hospital and in the ICU were 11 (range, 4-72) and 2 days (range, 1-3), respectively. The most common cause of surgery was bowel perforation (n=37, 44.0%), followed by intestinal obstruction (n=22, 26.2%). The small bowel was the most common operation site (n=43, 51.2%), followed by the colon (n=32, 38.1%). The most common type of surgery was segmental resection with primary anastomosis of the small bowel (n=33, 39.3%). Oral feeding was performed in 65 patients (77.4%), while tube feeding was performed in 19 patients (22.6%). Fifty-two patients (61.9%) experienced a postoperative complication, among which wound problems (infection or seroma) were the most common. Twenty-five patients (29.8%) experienced a pulmonary complication, consisting of atelectasis in eight, pneumonia in two, and pleural effusion in 15.
Group E comprised 44 patients (52.4%) and group L contained 40 patients (47.6%). In group E, 18 patients (40.9%) started feeding within 24 hours. Five patients (11.4%) were forced to stop feeding in group E (four cases of ileus, one anastomosis leakage), while 5 patients (12.5%) were forced to stop feeding in group L (four cases of ileus, two diarrhea).
No significant intergroup differences were found with respect to cause (Table 1), site (Table 2), type of operation (Table 3), gender, age, APACHE II score on ICU admission, gastric residual volume before feeding, ratio of vasopressor use, ICU care, and MV or duration of MV (Table 4). However, postoperative LOSs in the ICU and hospital were significantly longer in group L. The incidences of postoperative complications were not significantly different between the two groups (Table 5). However, pulmonary complications such as atelectasis, pneumonia and pleural effusion were significantly more common in group L. Five patients in group L were managed by percutaneous catheter drainage due to pleural effusion. However, no patient in group E was managed in this manner due to pleural effusion.
An intra-abdominal abscess developed in 2 patients (1 in each group), but both cases were well controlled by percutaneous catheter drainage. One patient in group E, who received pyloric exclusion and gastrojejunostomy due to duodenal perforation, required re-operation to treat anastomotic disruption, but recovered well after re-operation. No postoperative mortalities were recorded in the present study.
The present study focused on the safety of early feeding after emergency GI surgery in patients with relatively stable hemodynamic status and secure anastomosis. The findings of this study suggest that early feeding is safe after emergency GI surgery. Furthermore, complication rates were similar between the early and late groups; nevertheless, pulmonary complication rates were lower and LOSs in the hospital and the ICU were shorter in the early feeding group.
Traditionally, enteral feeding is not started until bowel motility has recovered after elective surgery on the GI tract,17 causing delays in enteral feeding after emergency surgery, compared with elective surgery. Because patients that undergo emergency GI surgery have an edematous or ischemic bowel, anastomosis healing is usually delayed, and this can result in anastomotic disruption or leakage. On the other hand, poor enteral intake can lead to malnutrition or delayed bowel mucosa growth and increase postoperative morbidity and mortality.
Several studies have reported beneficial effects for early enteral feeding after GI surgery, and demonstrated good tolerance to enteral feeding and reductions in septic morbidity.18,19 Whenever bowel continuity is maintained after surgery, enteral feeding is preferred over parenteral nutrition according to several guidelines.20,21 However, despite the beneficial effects of early enteral feeding, the timing of feeding commencement after emergency GI surgery remains controversial. Furthermore, few studies have addressed the beneficial effects of early enteral feeding after emergency GI surgery.12,22 A previous report on early enteral feeding after emergency GI surgery focused on patients with peritonitis.6 However, most of the patients enrolled had a perforated gastric or duodenal ulcer, and thus, feeding materials were not passed through anastomosis sites because a naso-gastric or percutaneous jejunal tube that passed through anastomosis was used for feeding. However, our patients had undergone bowel resection with anastomosis, and most (67%) were fed per os or through a naso-gastric tube positioned in the stomach.
Herein, complications associated with early feeding, such as abdominal pain, diarrhea, and postoperative ileus were investigated. Although complications developed in 23 of 44 patients in the early feeding group, all recovered fully under conservative management. As well, the majority of complications were wound problems, such as infection or seroma, and no differences were found between the two groups, with the exception of pulmonary complications.
In regards to pulmonary complications after emergency surgery, Barlow, et al.11 demonstrated that operative morbidity was less common after major upper GI surgery in patients that received early enteral nutrition. In particular, chest infections were significantly less common in these patients. Moore, et al.,18 via meta-analysis of high-risk surgical patients, also found that early enteral feeding was associated with a lower incidence of pneumonia and other septic complications. In accordance with previous studies, the present study showed that pulmonary complications were significantly less common in the early feeding group (the majority of pulmonary complications were due to pleural effusion and were treated by percutaneous catheter drainage). Additionally, it appeared to us that percutaneous catheter drainage for pleural effusion prolonged LOSs in the late feeding group, suggesting that early feeding resulted in better fluid balance; however, this was not evaluated. It was also noted that after feeding had started, intravenous fluid intake was reduced, suggesting that early enteral feeding could reduce the risk of pleural effusion.
Early enteral feeding is not routinely undertaken after emergency gastrointestinal surgery. Early in this study we started enteral feeding after flatus. However, after adaptation of ESPEN guidelines, feeding methods and timing were changed to start feeding as soon as possible after stabilization or to use an enteral tube catheter in severe or upper gastrointestinal surgery.
There are some limitations to this study. First, this study was retrospective in design and selection bias may be present. As well, the early feeding group may have had faster GI motility recovery and enjoyed a shorter LOS than the late feeding group. However, early feeding was started when the patient demonstrated hemodynamically stable status and secure bowel anastomosis was performed, rather than according to the GI motility of the patients. Consequently, there was no intergroup difference in gastric residual volume before feeding in our study. Second, the early enteral feeding group showed reduced postoperative LOSs in the ICU (group E vs. group L; 1 day vs. 2 days). However, early feeding was defined as commencement of diet within 48 hours after surgery in our study. Although 40.9% of patients started feeding within 24 hours after surgery, some patients were probably transferred to the general ward before feeding commencement. Thus, the impact of early feeding on reducing LOSs in the ICU requires further research, including severely-ill patients with long LOSs in the ICU. Third, our study was unable to assess the effects of early feeding on nutrition and fluid balance. Therefore, we suggest that a prospective study be undertaken to confirm the beneficial effects of early feeding after emergency GI surgery on nutrition and fluid balance.
In conclusion, this study showed that complication rates were similar between early and late feeding groups after emergency GI surgery. Our results indicate that early enteral feeding after emergency GI surgery does not increase complication rates, and thus, that early feeding after emergency GI surgery is feasible in patients without severe shock or bowel anastomosis instability.
ACKNOWLEDGEMENTS
Lee JG and Lee HS designed this study. And Lee HS, Shim H, Jang JY, and Lee H collected the data. Analysis and interpretation of data was performed by Lee HS and Shim H. Lee HS wrote the manuscript, and all of authors contributed to the revision. Critical revision was performed by Lee JG.
References
1. Bisgaard T, Kehlet H. Early oral feeding after elective abdominal surgery--what are the issues? Nutrition. 2002; 18:944–948.

2. Fearon KC, Luff R. The nutritional management of surgical patients: enhanced recovery after surgery. Proc Nutr Soc. 2003; 62:807–811.

3. Giner M, Laviano A, Meguid MM, Gleason JR. In 1995 a correlation between malnutrition and poor outcome in critically ill patients still exists. Nutrition. 1996; 12:23–29.

4. Pennington CR, Powell-Tuck J, Shaffer J. Review article: artificial nutritional support for improved patient care. Aliment Pharmacol Ther. 1995; 9:471–481.
5. Hill GL, Blackett RL, Pickford I, Burkinshaw L, Young GA, Warren JV, et al. Malnutrition in surgical patients. An unrecognised problem. Lancet. 1977; 1:689–692.
6. Kaur N, Gupta MK, Minocha VR. Early enteral feeding by nasoenteric tubes in patients with perforation peritonitis. World J Surg. 2005; 29:1023–1027.

7. Pupelis G, Selga G, Austrums E, Kaminski A. Jejunal feeding, even when instituted late, improves outcomes in patients with severe pancreatitis and peritonitis. Nutrition. 2001; 17:91–94.

8. Daly JM, Lieberman MD, Goldfine J, Shou J, Weintraub F, Rosato EF, et al. Enteral nutrition with supplemental arginine, RNA, and omega-3 fatty acids in patients after operation: immunologic, metabolic, and clinical outcome. Surgery. 1992; 112:56–67.
9. Dissanaike S, Pham T, Shalhub S, Warner K, Hennessy L, Moore EE, et al. Effect of immediate enteral feeding on trauma patients with an open abdomen: protection from nosocomial infections. J Am Coll Surg. 2008; 207:690–697.

10. Lassen K, Soop M, Nygren J, Cox PB, Hendry PO, Spies C, et al. Consensus review of optimal perioperative care in colorectal surgery: Enhanced Recovery After Surgery (ERAS) Group recommendations. Arch Surg. 2009; 144:961–969.

11. Barlow R, Price P, Reid TD, Hunt S, Clark GW, Havard TJ, et al. Prospective multicentre randomised controlled trial of early enteral nutrition for patients undergoing major upper gastrointestinal surgical resection. Clin Nutr. 2011; 30:560–566.

12. Singh G, Ram RP, Khanna SK. Early postoperative enteral feeding in patients with nontraumatic intestinal perforation and peritonitis. J Am Coll Surg. 1998; 187:142–146.

13. Malhotra A, Mathur AK, Gupta S. Early enteral nutrition after surgical treatment of gut perforations: a prospective randomised study. J Postgrad Med. 2004; 50:102–106.
14. Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. 1985; 13:818–829.
15. Bone RC, Balk RA, Cerra FB, Dellinger RP, Fein AM, Knaus WA, et al. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Chest. 1992; 101:1644–1655.

16. O'Keefe SJ, Buchman AL, Fishbein TM, Jeejeebhoy KN, Jeppesen PB, Shaffer J. Short bowel syndrome and intestinal failure: consensus definitions and overview. Clin Gastroenterol Hepatol. 2006; 4:6–10.
17. Wilmore DW, Long JM, Mason AD Jr, Skreen RW, Pruitt BA Jr. Catecholamines: mediator of the hypermetabolic response to thermal injury. Ann Surg. 1974; 180:653–669.
18. Moore FA, Feliciano DV, Andrassy RJ, McArdle AH, Booth FV, Morgenstein-Wagner TB, et al. Early enteral feeding, compared with parenteral, reduces postoperative septic complications. The results of a meta-analysis. Ann Surg. 1992; 216:172–183.

19. Lewis SJ, Egger M, Sylvester PA, Thomas S. Early enteral feeding versus "nil by mouth" after gastrointestinal surgery: systematic review and meta-analysis of controlled trials. BMJ. 2001; 323:773–776.

20. Braga M, Ljungqvist O, Soeters P, Fearon K, Weimann A, Bozzetti F. ESPEN Guidelines on Parenteral Nutrition: surgery. Clin Nutr. 2009; 28:378–386.

21. Bankhead R, Boullata J, Brantley S, Corkins M, Guenter P, Krenitsky J, et al. Enteral nutrition practice recommendations. JPEN J Parenter Enteral Nutr. 2009; 33:122–167.




 PDF
PDF ePub
ePub Citation
Citation Print
Print






 XML Download
XML Download