Abstract
Purpose
To evaluate the risk factors of hepatocellular carcinoma (HCC) extension into the right atrium (RA) and determine poor prognostic factors for HCC extension to the heart.
Materials and Methods
A total of 665 patients who were newly diagnosed with HCC were analyzed retrospectively from January 2004 to July 2012. The patients were divided into two groups: 33 patients with HCC extending into the RA and 632 HCC patients during the same period. The patients with HCC extending into the RA were subdivided into shorter survival group (<2 months) and longer survival group (≥2 months).
Results
The prevalence of HCC extending to the RA was 4.96%. In multivariate analysis, a modified Union Internationale Contre le Cancer (UICC) stage higher than IVA, hepatic vein invasion, concomitant inferior vena cava and portal vein invasion, and multinodular tumor type were risk factors for HCC extending to the RA. In multivariate analysis, Cancer of the Liver Italian Program (CLIP) score >3 (p=0.016, OR: 13.89) and active treatment (p=0.024, OR: 0.054) were associated with prognostic factors in patients HCC extending into the RA. Active treatment such as radiation (n=1), transcatheter arterial chemoembolization (TACE) (n=11), Sorafenib (n=1), and combined modalities (n=2) were performed.
Hepatocellular carcinoma (HCC) is the seventh most common carcinoma worldwide and the third most common cause of cancer-related mortality.1 HCC is a highly malignant tumor with a propensity for invading intrahepatic vascular structures, the portal vein (PV), and the hepatic vein (HV). Infrequently, the tumor thrombi within the HV may extend into the inferior vena cava (IVC) and the right atrium (RA). The prevalence of intra-atrial tumor growth in autopsied cases of HCC ranges from 1 to 4.8%.2-4 With advances in imaging, intra-atrial tumor growth has been increasingly recognized in HCC patients.5-7 When HCC extends into the IVC or the heart, the prognosis is poor, with a median survival period of only 2-3 months.8 The prognosis of HCC patients with extrahepatic metastasis is unsatisfactory and often not well known. The median survival period is 4.9 months (range, 1-37 months).9
HCC extension into the heart can induce sudden pulmonary embolism, intractable heart failure, secondary Budd-Chiari syndrome, and ball-valve thrombosis syndrome, and all of these complications can cause sudden death.6,10
Aggressive surgical treatment and nonsurgical local treatment modalities11-15 are efficacious, although such procedures are usually prohibited in most patients with advanced HCC because of underlying cirrhosis and the advanced stage of the primary tumor. No established risk factors, prognostic factors or effective treatment strategies for HCC cases that extend into the heart have been reported to date. Chun, et al.16,17 reported that active treatment, such as radiation, systemic chemotherapy, and transcatheter arterial chemoembolization (TACE) beyond supportive care, may provide a survival benefit in patients with HCC that extends into the IVC/heart, but this study was limited by a small sample size.
Therefore, the current study aimed to evaluate the risk factors and predictive factors for poor prognosis of patients with HCC extending into the RA.
From January 2004 to December 2012, a total of 1042 patients with newly diagnosed HCC were admitted to the Division of Gastroenterology at Chonnam National University Hospital in Gwangju, Korea. Overall, 377 patients were excluded because laboratory data available at the end of the follow-up period were incomplete. Information for 665 patients with newly diagnosed HCC were evaluated retrospectively. Among these patients, 33 patients had RA invasion during HCC followed up. In total, 632 patients who were diagnosed during the same period served as the comparison group. To describe the risk factors of HCC extension into the RA and determine predictive factors for poor prognosis in patients with HCC extending into RA, the data collected from the medical records included demographics, laboratory results, tumor characteristics, tumor stage, imaging studies, treatment modalities, and overall survival.
The diagnosis of HCC was based on the following guidelines proposed by the Korea Liver Cancer Study Group and the National Cancer Center18: 1) nodules >2 cm in diameter with a typical pattern of HCC in one imaging study or alpha-fetoprotein (AFP) levels >200 ng/mL and 2) nodules between 1 and 2 cm in diameter with a coincidental typical vascular pattern in two imaging studies. If these criteria were not met, biopsies were performed. Clinical staging was based on the modified Union Internationale Contre le Cancer (UICC) tumor-node-metastasis classification and Cancer of the Liver Italian Program (CLIP) scores.18,19 RA invasion was defined as direct invasion from the liver into the RA or intra-cardiac tumor thrombosis and diagnosed using CT, MRI, positron emission tomography, or echocardiography during follow-up of HCC and new symptom development, such as aggravated dyspnea, ascites, and chest discomfort. HCC with RA invasion was commonly diagnosed using CT. On imaging, the RA embolus was commonly observed as having a regular, slightly irregular round, orbicular-ovate, or lobulated type. In the arterial phase, the embolus demonstrated slight enhancement, whereas in the portal phase and the delayed phase, the contrast media were partially discharged.20
Medical chart review showed that 18 patients received supportive care and 15 patients received active treatment according to the individual situation such as economic state, Eastern Cooperative Oncology Group score, and patient's will to treat. To evaluate whether active treatment was superior to supportive care alone in terms of the overall survival of patients, we stratified 33 patients into the following two groups: a supportive care group (n=18) and an active treatment group (n=15). All of the patients were followed through December 2012.
According to Chern, et al.,8 when HCC extends into the IVC or the heart, median survival period is only 2-3 months.Therefore, we defined poor prognosis group as survival time shorter than 2 months.
Written informed consent was obtained from all of the patients regarding the nature and the purpose of the treatment, and this study was approved by the IRB of our institution.
Continuous variables were expressed as mean±standard deviation. A Student's t-test and Pearson's chi-squared test or Mann-Whitney test were used to compare the baseline characteristics of the patients. Factors that were significant in the univariate analysis were entered into a stepwise multivariate analysis to determine which risk factors retained statistical significance and which factors were dependent on other factors. The survival times were estimated from the date of HCC diagnosis until death. Null hypotheses of no differences were rejected if the p values were less than 0.05 or, equivalently, if the 95% CI of the odds ratio estimates excluded 1. We performed statistical analysis using SPSS 20.0 (SPSS Inc., IBM Company, Chicago, IL, USA).
The median duration of follow-up was 857 days (2-3906 days). The mean age of the patients was 59.7 years (range 32-87 years), and there were 561 men (84.4%) and 104 women (15.6%) included in the study. Overall, 373 patients (56.1%) were positive for hepatitis B surface antigen, 105 patients (15.8%) were positive for antibodies against hepatitis C virus (anti-HCV Ab), 121 patients (18.2%) were chronic alcohol drinkers, 36 patients (5.4%) had a combined etiology, and 30 patients (4.5%) had an unknown etiology. Regarding treatment method, 433 patients (65.1%) underwent TACE, 83 (12.5%) RFA, 76 (11.4%) surgery, 2 (0.3%) radiation therapy (RT), 16 (24.1%) Sorafenib, 6 (0.9%) hepatic arterial infusion chemotherapy, and 49 (7.3%) supportive care. There were significant differences between the characteristics of the two groups (HCC with RA invasion vs. newly diagnosed HCC during the same period), including the modified UICC stage, the CLIP score, the Child-Pugh score, the serum C-reactive protein (CRP) levels, PV invasion, HV invasion, IVC invasion, tumor size, tumor type, and the duration of follow-up. The clinical characteristics of the two groups are shown in Table 1.
A total of 13 patients (39.3%) had aggravated abdominal distension and/or dyspnea, 11 patients (33.3%) had non-specific symptoms (fatigue or poor oral intake), 8 patients (24.2%) had aggravated right upper quadrant pain, and 1 patient (3%) had chest discomfort on diagnosis of HCC with RA invasion. Suspicious right heart failure signs, such as aggravated ascites/dyspnea (39.3%), were the dominant symptoms.
Of a total of 665 patients with newly diagnosed HCC in our institution, 33 patients (4.96%) had RA invasion during HCC follow-up. In the univariate analysis, a modified UICC stage greater than IVA, a CLIP score >4, serum CRP levels >0.8 mg/dL, PV invasion, HV invasion, concomitant IVC and PV invasion, a tumor size ≥5 cm, a multinodular tumor type, and lymph node involvement were risk factors for HCC with RA invasion.
In the multivariate analysis, a modified UICC stage greater than IVA, HV invasion, concomitant IVC and PV invasion, and a multinodular tumor type were identified as independent risk factors (Table 2).
According to survival time, 9 patients survived less than 2 months, 24 patients survived longer than 2 months. The patients with HCC extending into the RA were subdivided into shorter survival group (<2 months) and longer survival group (≥2 months). There were no significant differences between the characteristics of the two groups (shorter survival group vs. longer survival group) including gender, age, cause of HCC, antiviral treatment, the modified UICC stage, the Child-Pugh score, the serum CRP, AFP levels, HV invasion, IVC invasion, tumor size, tumor type, lymph node, distant metastasis except PV thrombosis (p=0.054), CLIP score >3 (p=0.057), and active treatment (p=0.07).
In multivariate analysis, CLIP score >3 (p=0.016, OR: 13.89) and active treatment (p=0.024, OR: 0.054) were associated with prognostic factors in patients HCC extending into the RA (Table 3). Regarding to treatment course of patients with longer survival >6 months, TACE (n=3), RT (n=1), TACE+RT (n=1), and Sorafenib (n=1) were performed.
The median duration of follow-up in patients with HCC that extended into the RA was 123 days (range 2-467 days) (Fig. 1). Survival curve of patients with HCC and RA invasion between active treatment and supportive care are shown in Fig. 2.
The mean age of these patients was 60.88 years (range 37-86 years), and there were 28 men (84.8%) and 5 women (15.2%). The mean CLIP score was 3.39 (range 1-6), and the mean Child-Pugh score was 6.46 (range 5-11). There were no significant differences between the two groups (active treatment vs. supportive care) according to age, CLIP scores, Child-Pugh scores, PV invasion, tumor size, tumor type (Table 4). The treatment modalities in the active treatment group, including radiation, TACE, Sorafenib, and combined modalities, were performed according to the indications or situation for each patient (Table 5). After active treatment, most of patients who had right heart failure sign and chest discomfort had more improvement of symptom.
The causes of mortality in the best supportive care group included hepatic failure (n=6/18, 33.3%), tumor progression (n=5/18, 27.7%), upper gastrointestinal bleeding (n=2/18, 11.1%), ruptured HCC (n=2/18, 11.1%), sepsis due to pneumonia (n=1/18, 5.5%), and unknown causes (n=2/18, 11.1%).
In the active treatment group, the causes of mortality included hepatic failure (n=3/14, 21.4%), tumor progression (n=1/14, 7.1%), upper gastrointestinal bleeding (n=4/14, 28.5%), ruptured HCC (n=3/14, 21.4%), and cardiac problems (n=3/14, 21.4%). One patient is still alive until last follow-up.
The clinical course of HCC with a tumor thrombus in the PV, IVC, or RA is dismal. The median survival times of patients with an IVC tumor thrombus have been reported to be as short as 2-3 months without effective treatment.22 Because cases of HCC extending into the IVC/heart are rare and occur at a prevalence of 1-4%,10,23 effective treatment strategies and prognosis for these patients remain unclear.24,25 A few large-scale clinical studies of patients with HCC extending into the IVC/heart have been published,8,16 and no research has been done regarding the risk factors and the outcomes for HCC extending into the RA.
In our retrospective study, risk factors/predictive factors for prognosis and treatment outcomes were assessed with univariate and multivariate analysis.
The current study showed that HCC that extends into the RA is a rare disease (4.96%).
A modified UICC stage greater than IVA, HV invasion, concomitant PV and IVC invasion, and multinodular HCC are risk factors for advanced HCC with RA invasion. A CLIP score ≤3, active treatment may prolong survival in patients with HCC extending into RA. To the best of our knowledge, this study is the first to characterize the risk factors and predictive factors for poor prognosis of HCC extending into the RA.
In an autopsy study of 439 HCC cases, Kojiro, et al.3 found intra-atrial tumor growth in 18 cases. In addition, a continuous tumor thrombus that involved the RA, the IVC, and the HV was observed in 15 cases, and the tumors were observed to cross the tricuspid valve and enter the ventricle in five cases. Consistent with the findings of another studies,3,20 our study found a prevalence of HCC with RA invasion of 4.96%, and HV and IVC invasion were observed in most cases. IVC invasion was observed in 100% of the HCC cases with RA invasion. In our study, a modified UICC stage greater than IVA, HV invasion, concomitant IVC and PV invasion, and a multinodular tumor type were independent risk factors for HCC that extended into the RA. Therefore, tumor seeding through the venous system may be the most important route for HCC with RA invasion. As for symptoms, 13 patients (39.3%) had aggravated abdominal distension and/or dyspnea, 11 patients (33.3%) had non-specific symptoms (fatigue or poor oral intake), 8 patients (24.2%) had aggravated right upper quadrant pain, and 1 patient (3%) had chest pain. Because right heart failure signs (39.3%) were the dominant symptoms in patients with HCC that extended into the heart, we suggest that heart involvement should be suspected when HCC patients with high risk factors develop right heart failure signs, such as dyspnea, aggravated ascites, and/or lower leg edema.
In agreement with Chern's study, our study found that the median survival time for HCC patients with RA invasion was 123 days (approximately 4.1 months).
In regard with predictive factors for poor prognosis, neither liver function nor tumor stage are associated with survival. No active treatment and CLIP score >3 are significantly associated with short-term mortality.
As for treatment modalities, the results of earlier studies on surgical resection for the treatment of HCC with a metastatic IVC and RA tumor thrombus are disappointing results, with a mean survival time of approximately 8 months.26 Recent studies have found that TACE is the most widely used and effective treatment in patients with advanced HCC with IVC/RA invasion, and this treatment has been shown to provide a survival benefit to selected patients.8,13,20 Oral thalidomide and external beam radiation therapy have also been effective in advanced HCC with IVC/RA tumor thrombi; however, these treatment modalities were evaluated in small scale studies.14,27 Chun, et al.16 reported that active treatment, such as radiation, systemic chemotherapy, and TACE beyond supportive care, provided a survival benefit in patients with HCC that extended into the IVC/heart. In consistent with other studies, our study demonstrated that the active treatment such as RT, TACE, and Sorafenib prolong survival of patients with HCC extending into RA. However, it was difficult to evaluate the efficacy of each treatment modality because of the small sample size. To date, no effective therapies have been advanced for the treatment of HCC with IVC and RA tumor thrombi. Therefore, further studies are required to develop treatment strategies for HCC with IVC and RA invasion.
This study had several limitations. First, this was a retrospective, single-center analysis. Second, we did not confirm whether the intra-cardiac masses indicated HCC invasion or a benign cardiac tumor, such as myxoma. However, the incidence of cardiac tumors is low (0.02%), therefore, this might not have greatly influenced the results. Furthermore, the intra-cardiac masses were consistent with HCC according to the radiological findings. Third, we had a small sample size for comparing the poor prognosis factors among the patients with HCC extending to RA invasion.
In conclusion, HCC that extends into the RA is a rare disease (4.96%), and the median survival time of the patients in our study was 123 days.
A modified UICC stage greater than IVA, HV invasion, concomitant PV and IVC invasion, and multinodular HCC are risk factors for advanced HCC with RA invasion. Active treatment beyond supportive care may prolong survival.
However, larger prospective studies are needed to confirm these findings.
Figures and Tables
 | Fig. 1The survival curve of patients with HCC extending into the RA. HCC, hepatocellular carcinoma; RA, right atrium. |
 | Fig. 2The survival curves of patients in the supportive care and active treatment groups with HCC and RA invasion (log rank test, p=0.037). |
Table 1
Clinical Characteristics of Each Group of Patients
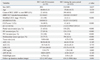
UICC, Union Internationale Contre le Cancer; CLIP, Cancer of the Liver Italian Program; IVC, Inferior vena cava; AFP, α-fetoprotein; CRP, C-reactive protein; HCC, hepatocellular carcinoma; RA, right atrium; HCV, hepatitis C virus; HV, hepatic vein; HBV, hepatitis B virus; AST, aspartate aminotransferase; ALT, alanine aminotransferase; LDH, lactate dehydrogenase.
ACKNOWLEDGEMENTS
This study is supported by grants from Chonnam National University Hospital, 42 Jebong-ro, Dong-gu, Gwangju 501-757, Korea.
References
1. Yang JD, Roberts LR. Hepatocellular carcinoma: a global view. Nat Rev Gastroenterol Hepatol. 2010; 7:448–458.

2. Edmondson HA, Steiner PE. Primary carcinoma of the liver: a study of 100 cases among 48,900 necropsies. Cancer. 1954; 7:462–503.

3. Kojiro M, Nakahara H, Sugihara S, Murakami T, Nakashima T, Kawasaki H. Hepatocellular carcinoma with intra-atrial tumor growth. A clinicopathologic study of 18 autopsy cases. Arch Pathol Lab Med. 1984; 108:989–992.
4. Kew MC, Paterson AC. Unusual clinical presentations of hepatocellular carcinoma. Trop Gastroenterol. 1985; 6:10–22.
5. Chua SO, Chiang CW, Lee YS, Liaw YF, Chang CH, Hung JS. Echocardiographic findings of mobile atrial hepatocellular carcinoma. Report of five cases. J Ultrasound Med. 1989; 8:347–352.

6. Lei MH, Ko YL, Kuan P, Lien WP, Chen DS. Metastasis of hepatocellular carcinoma to the heart: unusual patterns in three cases with antemortem diagnosis. J Formos Med Assoc. 1992; 91:457–461.
7. Ayoola EA, Olubuyide IO, Thomas J. Cardiovascular systemic invasion by hepatocellular carcinoma: incidence and pattern in a west African population. Afr J Med Med Sci. 1994; 23:61–66.
8. Chern MC, Chuang VP, Cheng T, Lin ZH, Lin YM. Transcatheter arterial chemoembolization for advanced hepatocellular carcinoma with inferior vena cava and right atrial tumors. Cardiovasc Intervent Radiol. 2008; 31:735–744.

9. Uka K, Aikata H, Takaki S, Shirakawa H, Jeong SC, Yamashina K, et al. Clinical features and prognosis of patients with extrahepatic metastases from hepatocellular carcinoma. World J Gastroenterol. 2007; 13:414–420.

10. Papp E, Keszthelyi Z, Kalmar NK, Papp L, Weninger C, Tornoczky T, et al. Pulmonary embolization as primary manifestation of hepatocellular carcinoma with intracardiac penetration: a case report. World J Gastroenterol. 2005; 11:2357–2359.

11. Goto H, Kaneko Y, Utoh J, Nishimura K, Miyauchi Y, Iwanaga K. Surgery of hepatoma with intracavitary cardiac extension. Heart Vessels. 1986; 2:60–62.

12. Saïsse J, Hardwigsen J, Castellani P, Caus T, Le Treut YP. Budd-Chiari syndrome secondary to intracardiac extension of hepatocellular carcinoma. Two cases treated by radical resection. Hepatogastroenterology. 2001; 48:836–839.
13. Kashima Y, Miyazaki M, Ito H, Kaiho T, Nakagawa K, Ambiru S, et al. Effective hepatic artery chemoembolization for advanced hepatocellular carcinoma with extensive tumour thrombus through the hepatic vein. J Gastroenterol Hepatol. 1999; 14:922–927.

14. Giuliani ME, Knox J, Dawson LA. Malignant intracardiac thrombus from hepatocellular carcinoma treated with external beam radiation therapy. J Palliat Med. 2010; 13:1293–1295.

15. Lin HH, Hsieh CB, Chu HC, Chang WK, Chao YC, Hsieh TY. Acute pulmonary embolism as the first manifestation of hepatocellular carcinoma complicated with tumor thrombi in the inferior vena cava: surgery or not? Dig Dis Sci. 2007; 52:1554–1557.

16. Chun YH, Ahn SH, Park JY, Kim do Y, Han KH, Chon CY, et al. Clinical characteristics and treatment outcomes of hepatocellular carcinoma with inferior vena cava/heart invasion. Anticancer Res. 2011; 31:4641–4646.
17. Kim SU, Kim YR, Kim do Y, Kim JK, Lee HW, Kim BK, et al. [Clinical features and treatment outcome of advanced hepatocellular carcinoma with inferior vena caval invasion or atrial tumor thrombus]. Korean J Hepatol. 2007; 13:387–395.

18. Park JW. Korean Liver Cancer Study Group and National Cancer Center. [Practice guideline for diagnosis and treatment of hepatocellular carcinoma]. Korean J Hepatol. 2004; 10:88–98.
19. A new prognostic system for hepatocellular carcinoma: a retrospective study of 435 patients: the Cancer of the Liver Italian Program (CLIP) investigators. Hepatology. 1998; 28:751–755.
20. Cheng HY, Wang XY, Zhao GL, Chen D. Imaging findings and transcatheter arterial chemoembolization of hepatic malignancy with right atrial embolus in 46 patients. World J Gastroenterol. 2008; 14:3563–3568.

21. Cha J, Seong J, Lee IJ, Kim JW, Han KH. Feasibility of sorafenib combined with local radiotherapy in advanced hepatocellular carcinoma. Yonsei Med J. 2013; 54:1178–1185.

22. Zeng ZC, Fan J, Tang ZY, Zhou J, Qin LX, Wang JH, et al. A comparison of treatment combinations with and without radiotherapy for hepatocellular carcinoma with portal vein and/or inferior vena cava tumor thrombus. Int J Radiat Oncol Biol Phys. 2005; 61:432–443.

23. Martínez Baca-López F, Ramírez-Arias E, Rayas-Gómez AL, Bernal-Ruiz EA, Saturno-Chiu G. Hepatocellular carcinoma with invasion into right cardiac cavities: report of a case and literature review. J Am Soc Echocardiogr. 2004; 17:192–194.

24. Jeong DS, Sung Kim J, Kim KH, Ahn H. Left atrial metastasis from hepatocellular carcinoma with liver cirrhosis. Interact Cardiovasc Thorac Surg. 2010; 11:703–705.

25. Mukai K, Shinkai T, Tominaga K, Shimosato Y. The incidence of secondary tumors of the heart and pericardium: a 10-year study. Jpn J Clin Oncol. 1988; 18:195–201.
26. Asahara T, Itamoto T, Katayama K, Nakahara H, Hino H, Yano M, et al. Hepatic resection with tumor thrombectomy for hepatocellular carcinoma with tumor thrombi in the major vasculatures. Hepatogastroenterology. 1999; 46:1862–1869.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download