Abstract
Purpose
To evaluate the technical feasibility and clinical outcome of bilateral uterine artery embolization (UAE) as a first-line therapeutic option for bleeding uterine arteriovenous malformation (AVM).
Materials and Methods
Between 2002 and 2012, 19 patients were diagnosed with acquired uterine AVM clinically and through imaging studies. The clinical characteristics, angiographic features, technical success rate of embolization, procedure-related complications, imaging, and clinical follow-up data were assessed. Clinical success was defined as immediate symptomatic resolution with disappearance of vascular abnormality on subsequent imaging studies.
Results
A total of 20 bilateral UAE, with or without embolization of extra-uterine feeders, were performed as the first-line treatment. Technical and clinical success rate was 90.0% (18/20) and 89.5% (17/19), respectively. Embolization was incomplete in two patients who had residual extra-uterine fine feeders to the AVM or a procedure-related complication (ruptured uterine artery); the former showed slow regression of the vascular malformation during the observation period, while the latter underwent a successful second bilateral UAE. Immediate clinical success was achieved in the remaining 17 patients after a single session and no recurrence of bleeding was found. Recovery to normal menstrual cycle was seen in all 17 patients with clinical success within one or two months, two of whom subsequently had uneventful intrauterine pregnancies carried to term.
Uterine arteriovenous malformations (AVM) are rare but life-threatening disorders that account for 1-2% of profuse female genital bleeding.1-3 The diagnosis of uterine AVM is usually made when there is unexpected, excessive, intermittent bleeding, particularly after delivery or surgical procedures performed on the uterus.1,2,4 A high index of suspicion for uterine AVM is important in such clinical settings because intrauterine procedures for gynecologic evaluation, such as hysteroscopy or dilatation and curettage (D&C) may inadvertently aggravate hemorrhage.
According to their etiology, uterine AVMs are classified into idiopathic (or primary) and acquired (or secondary) forms. Idiopathic uterine AVMs of the uterus are usually developmental anomalies that exhibit histologic similarities to AVMs found at other sites and sometimes show extensive extra-uterine involvement, requiring endovascular and/or percutaneous treatment according to the angiographic pattern.4,5 However, acquired uterine AVMs are a secondary condition associated with delivery, surgical procedures, malignancies such as endometrial and cervical carcinomas, gestational trophoblastic disease, and rarely with maternal exposure to diethylstilbestrol.2,6 Although the precise etiology of acquired uterine AVMs remains unknown, trophoblastic invasion or reactive angiogenesis following myometrial or endometrial injury secondary to iatrogenic intrauterine procedures or pregnancy-related changes have both been suggested.4,7,8 Transvaginal or abdominal ultrasonography together with a color Doppler study is usually performed as the primary diagnostic tool to assess patients with suspected uterine AVM. In addition, CT or MRI of the pelvis can be performed in order to assess the extent of malformation and to detect any parasitic feeding arteries.3,4
Uterine artery embolization (UAE) is a well-recognized treatment option for obstetric and gynecologic hemorrhage, and its technical and clinical efficacy has been shown in numerous reports, even in patients with massive, uncontrollable bleeding.3,9-12 UAE has a major advantage of preserving uterine function owing to the extensive collateral blood supply compared with surgical options.12-14 Despite the large number of observational studies and case reports in the literature,7,8,12,15-19 issues such as whether to perform unilateral versus bilateral UAE and whether to embolize extra-uterine feeding arteries including dominant draining vein are still debated. In this study, we evaluated the technical feasibility and clinical outcome of UAE as a first-line therapeutic option for bleeding uterine AVM.
This retrospective study was approved by our Institutional Review Board, and informed consent was waived. A retrospective review of patients, who underwent pelvic angiography between January 2002 and August 2012, was performed using our electronic medical records and picture archiving/communication system. In total, 443 patients underwent UAE during this period, of which 19 presented with acquired uterine AVM.
All patients in this study presented with uncontrollable profuse vaginal bleeding and were immediately referred for angiographic evaluation and treatment following transvaginal ultrasonography performed in the Obstetrics and Gynecology Department. Three patients had undergone an angiographic study after scheduled pelvic CT examination in order to evaluate the cause of their aggravated vaginal bleeding after D&C. In four patients, pelvic MR examination was performed following the initial transvaginal ultrasonography evaluation. The choice of imaging modality was made by the referring physician, and each modality was assessed for the possible extra-uterine involvement of the AVM and presence of nonuterine parasitic feeding arteries. Follow-up transvaginal ultrasonography was performed after UAE according to the physicians' discretion in all patients, whereas only one patient underwent a follow-up pelvic MR for persisting symptoms.
We accessed the right common femoral artery in every patient and a 5-Fr introducing angiographic sheath (Terumo Corporation, Tokyo, Japan) was placed. A 5-Fr Cobra catheter (Cook, Bloomington, IN, USA) was used to perform nonselective angiograms of the internal iliac arteries in order to achieve a general understanding of the vascular anatomy associated with the uterine AVM; the right internal iliac artery was selected after creating a Waltman loop with the Cobra catheter. In lateralized UVMs, i.e. UVMs located predominantly off the midline and toward one side, the dominant uterine artery ipsilateral to the lesion was initially super-selected using a microcatheter ranging from 2.0 to 2.4 Fr (Terumo Corporation, Tokyo, Japan). Particulate embolic materials such as gelatin sponge pledgets (Gelfoam; Pharmacia & Upjohn Co., Kalamazoo, MI, USA) or polyvinyl alcohol particles (Contour; Boston Scientific, Cork, Ireland) were commonly used, yet micro-coils were occasionally chosen. Aor-tograms were obtained after bilateral UAE in all patients in order to identify any extra-uterine blood supply.
We performed a thorough review of the patients' medical records and imaging studies to collect data regarding the clinical manifestations, results of obstetric/gynecologic evaluation, angiographic features of the AVM before and after embolization, details of the procedure, type of embolic material used, technical and clinical outcome after embolization, and procedure-related complications. We also assessed how many of these patients succeeded or failed to conceive during the follow-up period.
We evaluated the angiographic distribution of uterine AVMs and other angiographic features such as the possible presence of extra-uterine parasitic feeding arteries and a prominent draining vein.
Technical success was defined as the complete disappearance of angiographic staining of the AVM on post-embolization angiography and the absence of any procedure-related complications. Clinical success was defined as immediate resolution of vaginal bleeding without symptom recurrence and resolution of the AVM on subsequent imaging studies.
Complications were classified as major or minor according to the guidelines of the Society of Interventional Radiology Standards of Practice Committee.20 Major complications were defined as those requiring major therapy, necessitating an unplanned increase in the level of care or prolonged hospitalization (>48 hours), or resulting in permanent adverse sequelae or death. Minor complications were defined as those requiring no or only nominal therapy, including overnight admission for observation.
In total, 19 women (mean age, 32.3 years; age range, 21-42 years) underwent 20 UAE procedures at our hospital for the treatment of bleeding uterine AVM. All 19 patients had previously undergone gynecological procedures or obstetric events, such as D&C (n=14) or delivery (n=5). Presenting symptoms were intermittent or progressive vaginal bleeding. The mean time interval between these obstetric events and symptom presentation was 6 weeks. One patient who presented with intermittent vaginal bleeding four weeks after D&C had been diagnosed with gestational trophoblastic disease through imaging and serum beta-HCG level. This patient received two cycles of chemotherapy after which complete remission of gestational trophoblastic disease was confirmed through normalized serum beta-HCG results. However, a subsequent imaging study was performed due to recurrence of intermittent vaginal bleeding, which revealed AVM in the uterus.
Hypertrophied uterine arteries leading into the AVM were seen in each case. Seven patients demonstrated lateralized AVMs mainly supplied by ipsilateral uterine arteries, while feeders from the contralateral uterine arteries were relatively poor; these AVMs were categorized as uterine AVMs with unilaterality (Fig. 1). Causal events were D&C in all the patients of this group. In the remaining twelve patients, even arterial supply to the AVM from bilateral uterine arteries was noted; these AVMs were categorized as uterine AVMs with bilaterality. Their causal events were D&C (n=7) or delivery (n=5).
Early venous drainage from the AVM to pelvic veins was demonstrated in all patients. Relatively fast flow to pelvic veins was noted at AVM with unilaterality. Prominent dilatation with marked flow to the right ovarian vein was noted in one patient. AVMs were accompanied by pseudoaneurysms of various sizes, ranging from 0.8-1.8 cm, in four patients whose causal events were all D&C.
Technical success was achieved in 90.0% (18/20 procedures) of the UAE procedures, and immediate clinical success was noted in 89.5% (17/19 patients). The two cases of technical failure were associated with immediate clinical failure.
One of the technical failures was caused by incomplete embolization of the feeders to uterine AVM. In this patient, an abdominal aortogram performed after bilateral UAE showed residual staining of the AVM with additional feeders from the left ovarian artery. After successful embolization of the bilateral uterine arteries and the left ovarian artery, another angiogram revealed extremely fine, extra-uterine feeders arising from the anterior division of left internal iliac artery. The procedure was aborted after failed attempts to catheterize these fine feeders, thus resulted in incomplete embolization. However, complete resolution of vaginal bleeding and AVM at the 5-month follow-up transvaginal ultrasonography was evident using close clinical observation.
The other technical failure was associated with uterine artery rupture. In this patient, prominent venous drainage via the right ovarian vein was noted on the right internal iliac angiogram. Because embolic materials could reflux into the right ovarian vein, embolization of the right ovarian vein was carried out in an attempt to slow down blood flow through the AVM. After successful embolization of the venous side, inadvertent rupture of the ascending segment of right uterine artery, which had a very tortuous anatomy, occurred during guide wire manipulation. The ruptured artery was subsequently embolized using microcoils. Although the patient had both a decreased amount and frequency of vaginal bleeding after the first embolization procedure, second UAE was performed eight months later because of continuous, intermittent vaginal bleeding and evidence of residual AVM on a subsequent pelvic MR examination. Angiography demonstrated fine feeders from the right uterine artery, recanalization of the left uterine artery, and parasitis feeders from the inferior mesenteric artery, all of which were successfully embolized (Fig. 2). The second procedure was technically and clinically successful with resolution of the patient's symptoms and gradual disappearance of the AVM on the follow-up transvaginal ultrasonography up to 15 months following the second procedure.
The embolic materials used most often were particulate agents such as gelfoam pledgets (n=13) and polyvinyl alcohol particles (n=6). Gelfoam sheets were cut into pledgets of the desired size, usually 1-2 mm. The most commonly used sizes of polyvinyl alcohol particles were 100-300 µm and 300-500 µm. Microcoils were used in three patients to embolize the uterine artery, ovarian vein, or a ruptured uterine artery. Two of these patients also had microcoils and gelfoam applied to the proximal segments of the bilateral UAs to enhance occlusion.
Only one procedure-related complication occurred, which was the aforementioned case with uterine artery rupture during wire manipulation. The diagnosis of a ruptured uterine artery was made after detecting contrast extravasation on angiography, and subsequent embolization of the ruptured artery was successfully performed using microcoils. No post-embolization syndrome or infection following embolization occurred.
Patients with clinical success showed no symptoms of recurrence during the follow-up period until the end of the study (mean: 52 months, range: 4-94 months). Recovery to their normal menstrual cycle was seen in all 17 patients, including two patients with technical failure, one to two months following UAE. Two patients achieved full-term pregnancy with uneventful labor. No other patients have become pregnant, nor have attempted to conceive (Fig. 3).
Imaging assessment of uterine AVMs, especially their vascular anatomy, is very important during the planning stages before embolization, such as Doppler ultrasonography, CT, and MRI.3,4,7,8 In our study, the lateralized uterine AVMs were related to iatrogenic injuries such as D&C and their dominant vascular feeders were from the ipsilateral uterine artery. In addition, all four patients with pseudoaneurysms had histories of D&C.18 Thus, we are able to assume from our results that acquired uterine AVMs are frequently related to iatrogenic injury. For example, D&C tends to be located laterally on either side with predominant feeder from the ipsilateral uterine artery, and is possibly accompanied by pseudoaneurysms. However, uterine AVMs associated with delivery (n=5) or gestational trophoblastic disease (n=1) showed predomiant feeders from bilateral uterine arteries. This finding supports the pathogenesis described in the literature that relates the development of AVMs to failed regression of proliferated vascular structures of pregnancy or abnormally developed vascular structures related to gestational trophoblastic disease.7,11,21
We advocate that embolization of bilateral UAs is superior to embolization of unilateral uterine artery. In all of our cases, regardless of the location or predominant feeding artery of the AVM, bilateral UAE was performed. By embolizing the predominant feeding uterine artery followed by the contralateral uterine artery, we expected a more secure and complete result from the embolization procedure. With only a single session of UAE where bilateral UAs were embolized, 17 patients showed complete resolution of uterine AVMs and no recurrence during follow-up. There were no extra-uterine feeders to the AVMs in these patients. With unilateral embolization, there is a potential for the contralateral non-dominant uterine artery to develop into a major feeding artery. In the absence of extra-uterine feeders, complete resolution of uterine AVMs is expected by bilateral UAE.
Pre-procedural imaging evaluation is necessary in order to assess the presence of possible extra-uterine feeders. An example is the ovarian-uterine anastomotic connection, which is often detected when performing UAE for uterine fibroids.22,23 It is technically challenging to embolize fine extra-uterine feeders, as we found in one patient of our study. This patient was closely observed after failed attempts at embolization of fine feeders from the anterior division of internal iliac artery; however, complete resolution of the AVM was seen on a follow-up imaging study. This clinical observation suggests that conservative management rather than aggressive intervention or surgical strategy could be a reasonable option when the UAE procedure provide insufficient angiographic results.7,9,24-27
In one of our patients with an AVM that was associated gestational trophoblastic disease, one draining ovarian vein that was markedly prominent was embolized with coils to reduce blood flow. To our knowledge, this is the first time such a case has been described in the literature, and we believe that embolization of the dominant draining vein is an important step in achieving effective embolization. This case corresponds to the type II arteriolovenous shunt, previously described by Cho, et al.,28 where multiple arterioles drain into a single venous component. In this patient, embolization of bilateral UAs and the inferior mesenteric artery was performed in the second session eight months following the first session. Considering the rich vascular collateral network of the uterus, thorough angiographic assessment is necessary to identify all existing extra-uterine feeders.
We advocate the safety of bilateral UAE for the management of acquired uterine AVMs where preservation of the uterine function is a major advantage of the procedure. The choice of embolic material, whether used independently or in combination, did not affect the clinical outcomes of UAE. According to our study, the use of gelfoam pledgets without the complementary use of any other embolic material seems effective in the resolution of acquired uterine AVMs. In addition, a three- to five-week duration of occlusion after bilateral UAE using gelfoam pledgets seemed sufficient to prevent further bleeding while still permitting the slow development of various collateral vasculature, thereby preventing ischemia.6,13,14,29,30 Menstrual cycles were restored within two months in all patients with clinically successful outcome. Two patients also had successful labors twelve months after their procedures. These results are compatible with those described in previous studies.12,15,24 Although, hypovascularity of the treated area may theoretically have adverse affects on placentation and fetal growth,31 normal adaptation of the uteroplacental vasculature after UAE is a plausible explanation for the restoration of the menstrual cycle and the potential for successful labor.
Our study has several limitations. First, this retrospective design possesses some inherent flaws; furthermore, the small sample size does not permit extensive statistical analysis. Second, the follow-up protocol was not consistent in all patients, and no images were available at long-term follow-up. Third, various angiographic devices and embolic materials were used for a long period.
In conclusion, bleeding uterine AVMs can be successfully managed by bilateral UAE, which is a safe and effective first-line therapeutic option. However, technical difficulties during the procedure may result in incomplete embolization in suboptimal clinical outcomes.
Figures and Tables
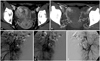 | Fig. 1A 26-year-old patient (No. 4) presenting with progressive vaginal bleeding. The axial enhanced CT scan (A) and CT angiography (B) show focal a hypervascular lesion (arrows) with feeders from the right uterine artery. (C and D) Right internal iliac arteriograms show the AVM along with the early opacification of the right ovarian vein. (E) Left internal iliac arteriogram does not show much blood supply to the AVM. Bilateral uterine arteries were embolized with gelfoam pledgets (not shown). At one-month follow-up, transvaginal ultrasound (not shown) showed resolution of the AVM together with clinical improvement. AVM, arteriovenous malformation. |
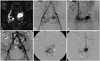 | Fig. 2A 21-year-old patient (No. 10) presenting with progressive vaginal bleeding. (A) The enhanced coronal MR image shows tortuosity of the right uterine artery with a large pseudoaneurysm (arrows) at the uterine fundus. (B and C) Aortograms show tortuosity of bilateral (the right side being dominant) uterine arteries, a pseudoaneurysm (arrows), and early drainage into the right ovarian vein (arrowheads). The right uterine artery was ruptured during guide wire manipulation. The right ovarian vein and ruptured right uterine artery were embolized using coils. (D) The angiogram obtained upon completion shows the remaining pseudoaneurysm. (E) Selective angiograms of the right uterine artery angiogram of the left uterine artery obtained eight months after the first embolization procedure show the residual AVMs with opacification of the pseudoaneurysm. (F) The selective angiogram of the inferior mesenteric artery angiogram shows the AVM with a pseudoaneurysm. All feeding arteries were completely embolized using polyvinyl alcohol particles and gelfoam pledgets (not shown). AVM, arteriovenous malformation. |
References
1. Aziz N, Lenzi TA, Jeffrey RB Jr, Lyell DJ. Postpartum uterine arteriovenous fistula. Obstet Gynecol. 2004; 103(5 Pt 2):1076–1078.

2. Fleming H, Ostor AG, Pickel H, Fortune DW. Arteriovenous malformations of the uterus. Obstet Gynecol. 1989; 73:209–214.
3. Flynn MK, Levine D. The noninvasive diagnosis and management of a uterine arteriovenous malformation. Obstet Gynecol. 1996; 88(4 Pt 2):650–652.

4. Cura M, Martinez N, Cura A, Dalsaso TJ, Elmerhi F. Arteriovenous malformations of the uterus. Acta Radiol. 2009; 50:823–829.

5. Arora R, Achla B, Pinkee S, Purba G, Bharti M. Arteriovenous malformations of the uterus. N Z Med J. 2004; 117:U1182.
6. Schiller VL, Raft E, Linden R. Uterine arteriovenous malformation. AJR Am J Roentgenol. 1998; 170:219–220.

7. Timmerman D, Van den Bosch T, Peeraer K, Debrouwere E, Van Schoubroeck D, Stockx L, et al. Vascular malformations in the uterus: ultrasonographic diagnosis and conservative management. Eur J Obstet Gynecol Reprod Biol. 2000; 92:171–178.

8. O'Brien P, Neyastani A, Buckley AR, Chang SD, Legiehn GM. Uterine arteriovenous malformations: from diagnosis to treatment. J Ultrasound Med. 2006; 25:1387–1392.
9. Brown JV 3rd, Asrat T, Epstein HD, Oglevie S, Goldstein BH. Contemporary diagnosis and management of a uterine arteriovenous malformation. Obstet Gynecol. 2008; 112(2 Pt 2):467–470.

10. Lin AC, Hung YC, Huang LC, Chiu TH, Ho M. Successful treatment of uterine arteriovenous malformation with percutaneous embolization. Taiwan J Obstet Gynecol. 2007; 46:60–63.

11. Lim AK, Agarwal R, Seckl MJ, Newlands ES, Barrett NK, Mitchell AW. Embolization of bleeding residual uterine vascular malformations in patients with treated gestational trophoblastic tumors. Radiology. 2002; 222:640–644.

12. Ghai S, Rajan DK, Asch MR, Muradali D, Simons ME, TerBrugge KG. Efficacy of embolization in traumatic uterine vascular malformations. J Vasc Interv Radiol. 2003; 14:1401–1408.

13. Amagada JO, Karanjgaokar V, Wood A, Wiener JJ. Successful pregnancy following two uterine artery embolisation procedures for arteriovenous malformation. J Obstet Gynaecol. 2004; 24:86–87.

14. Chia YN, Yap C, Tan BS. Pregnancy following embolisation of uterine arteriovenous malformation--a case report. Ann Acad Med Singapore. 2003; 32:658–660.
15. Peitsidis P, Manolakos E, Tsekoura V, Kreienberg R, Schwentner L. Uterine arteriovenous malformations induced after diagnostic curettage: a systematic review. Arch Gynecol Obstet. 2011; 284:1137–1151.

16. Guo N, Liu H, Peng Z. Uterine arteriovenous fistula necessitating hysterectomy after two unsuccessful embolizations in an 18-year-old patient. Ann Vasc Surg. 2010; 24:827.

17. Wang Z, Chen J, Shi H, Zhou K, Sun H, Li X, et al. Efficacy and safety of embolization in iatrogenic traumatic uterine vascular malformations. Clin Radiol. 2012; 67:541–545.

18. Kwon JH, Kim GS. Obstetric iatrogenic arterial injuries of the uterus: diagnosis with US and treatment with transcatheter arterial embolization. Radiographics. 2002; 22:35–46.

19. Maleux G, Timmerman D, Heye S, Wilms G. Acquired uterine vascular malformations: radiological and clinical outcome after transcatheter embolotherapy. Eur Radiol. 2006; 16:299–306.

20. Sacks D, McClenny TE, Cardella JF, Lewis CA. Society of Interventional Radiology clinical practice guidelines. J Vasc Interv Radiol. 2003; 14(9 Pt 2):S199–S202.

21. Vaknin Z, Sadeh-Mefpechkin D, Halperin R, Altshuler A, Amir P, Maymon R. Pregnancy-related uterine arteriovenous malformations: experience from a single medical center. Ultraschall Med. 2011; 32:Suppl 2. E92–E99.

22. Pelage JP, Walker WJ, Le Dref O, Rymer R. Ovarian artery: angiographic appearance, embolization and relevance to uterine fibroid embolization. Cardiovasc Intervent Radiol. 2003; 26:227–233.

23. Razavi MK, Wolanske KA, Hwang GL, Sze DY, Kee ST, Dake MD. Angiographic classification of ovarian artery-to-uterine artery anastomoses: initial observations in uterine fibroid embolization. Radiology. 2002; 224:707–712.

24. Dar P, Karmin I, Einstein MH. Arteriovenous malformations of the uterus: long-term follow-up. Gynecol Obstet Invest. 2008; 66:157–161.

25. Itoh H, Keitoku M, Fukuoka M, Sagawa N, Mori T, Togashi K. Spontaneous resolution of a postcesarean arteriovenous fistula of the uterine cervix: the usefulness of transvaginal color Doppler scanning. J Obstet Gynaecol Res. 1997; 23:439–444.

26. Forssman L, Lundberg J, Scherstén T. Conservative treatment of uterine arteriovenous fistula. Acta Obstet Gynecol Scand. 1982; 61:85–87.

27. Nonaka T, Yahata T, Kashima K, Tanaka K. Resolution of uterine arteriovenous malformation and successful pregnancy after treatment with a gonadotropin-releasing hormone agonist. Obstet Gynecol. 2011; 117(2 Pt 2):452–455.

28. Cho SK, Do YS, Shin SW, Kim DI, Kim YW, Park KB, et al. Arteriovenous malformations of the body and extremities: analysis of therapeutic outcomes and approaches according to a modified angiographic classification. J Endovasc Ther. 2006; 13:527–538.

29. Badawy SZ, Etman A, Singh M, Murphy K, Mayelli T, Philadelphia M. Uterine artery embolization: the role in obstetrics and gynecology. Clin Imaging. 2001; 25:288–295.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download