Abstract
Purpose
Myocardial infarction in children with total occlusion of a coronary artery after Kawasaki disease is rare due to multiple collateral vessels. We aimed to investigate the changes in coronary perfusion associated with coronary artery occlusion after Kawasaki disease.
Materials and Methods
Eleven patients with coronary artery occlusion after Kawasaki disease were investigated. Serial coronary angiographies after total occlusion of a coronary artery were reviewed and the changes were described in all patients with additive information collected.
Results
The median age at the occlusion was 5.9 years old. The interval to occlusion was 6.2±6.9 years. Four left anterior descending coronary artery total occlusions and 10 right coronary artery total occlusions were detected. Immediate coronary artery bypass graft for left anterior descending coronary artery total occlusion made right coronary total occlusion occurred in all except one patient and the intervals thereof were 1 year, 1.8 years, and 4 years. Collaterals to the left coronary artery regressed after recanalization, while new collaterals to the right coronary artery developed. In three, collaterals to the right coronary artery decreased without recanalization without clinical signs.
Approximately 5% of patients with Kawasaki disease (KD) have aneurysms of the coronary arteries despite receiving intravenous gamma-globulin therapy.1 Coronary artery aneurysms tend to regress, but sometimes, lead to stenosis or total occlusion, especially in patients with giant coronary aneurysm.2 Patients with severe coronary artery stenosis or obstruction due to KD frequently develop multiple collateral vessels.3 For this potential reason, patients with coronary stenosis or obstruction remain asymptomatic.4 Nevertheless, patients with clinical symptoms of acute myocardial infarction and revascularization require relief from symptoms.5,6 It has been proposed that collateral vessels develop more easily in the immature heart with ischemia, and accordingly, collateral vessels in coronary stenosis are found more often in children than adults.7 The development of collateral vessels associated with total occlusion of coronary artery is helpful to prevent myocardial infarction in infants and young children, and this may contribute to a better prognosis.8 Therefore, the status of collaterals associated with total occlusion of coronary artery in KD is important when deciding on a particular treatment. While there are a few reports on changes in myocardial perfusion after total occlusion of a coronary artery in KD patients, this study aimed to assess changes in patterns of myocardial perfusion after occlusion of coronary arteries in KD.
We reviewed all coronary angiographies (CAG) in patients diagnosed with KD at Samsung Medical Center from April 1994 to December 2010. The subjects consisted of patients who exhibited total occlusion of one or more coronary arteries that were confirmed by CAG. The patients who did not undergo CAG after diagnosis of total occlusion were excluded. For every patient, two-dimensional (2D) echocardiography was performed before every CAG. From the echocardiography, global ventricular function and regional wall motion were evaluated. Left ventricular shortening fraction from 28% to 38% was regarded as normal. Right ventricular function and regional wall motion abnormality of left ventricle were evaluated qualitatively by the investigator. 201Tl single photon emission computed tomography (SPECT) was checked at the time of the first diagnosis of total occlusion of coronary artery on CAG. The extent and the area of infarct were determined on the basis of the normal values of circumferential profiles using conventional methods. A 17-segment analysis with 5-point scoring was used for SPECT image interpretation. Qualitative visual analysis was performed by a specialist in cardiac nuclear medicine. The first CAG was performed after the confirmation of the coronary abnormality proven by echocardiography, usually at 6-9 months after KD. Follow-up CAG was performed at an interval of 1 to 3 years according to lesion severity, even those without any signs of myocardial ischemia. Selective CAG was performed for determining the location of occlusion and the degree of collateral vessels. In addition to occlusion, giant aneurysm greater than 8 mm in diameter, as well as stenosis (75-90%) or severe stenosis (>90%), was determined. We considered giant aneurysm to be at a very high risk of obstruction even without stenosis. The subjects comprised 1974 patients with KD who were consecutively admitted to our hospital. During the follow-up period, serial coronary angiography was performed in 67 patients. Total occlusion of coronary artery with collaterals was found in 12 patients. Eleven patients who underwent CAG more than twice were enrolled in this study. For these enrolled patients, medical records and 38 serial CAGs were reviewed in order to investigate the changes in coronary perfusion including collaterals after coronary artery occlusion. Occlusion time was defined as the age of the first detection of total occlusion of one or more of coronary arteries on CAG. The last follow-up age was the age at the last CAG. We determined serial changes in distal perfusion according to flow grading of coronary vessels and collateral blood flow according to flow grading of collateral vessels.
All variables are reported as ranges (median) and mean±standard deviation.
This study protocol was reviewed and approved by our Institutional Review Board, and informed consent for this research project was waived.
The classification proposed and validated by Cohen and Rentrop11 was used for assessment of collateral blood flow.
Among the eleven patients included for analysis, seven (63.6%) were male. The age at onset of KD ranged from 3 months to 7 years (median, 2.2 years old). The age at the occlusion ranged from 1.5 years old to 25.2 years old (median, 5.9 years old), and the follow-up duration till last CAG was 4.5±2.5 years. The interval from the onset of KD to the occlusion time was 6.2±6.9 years (1.5-20.8 years). Two patients (18.2%) complained of chest pain and significant ST-T change on electrocardiography was found in two patients (18.2%) when the occlusion was detected. A positive result on SPECT that was compatible with CAG was recorded in seven patients (63.6%). In all four patients with left anterior descending coronary artery (LAD) total occlusion (TIMI gr. 0), SPECTs showed compatible ischemic sign, whereas only three patients in six patients with isolated right coronary artery (RCA) total occlusion (TIMI gr. 0) had positive SPECT results. After recanalization, myocardial ischemia was recovered by SPECT in all patients. In three cases of consequent RCA total occlusion after LAD recanalization, SPECT was performed in only one patient and showed no significant change. Echocardiographically, only two patients showed abnormal findings of decreased left ventricular shortening fractions (13% and 20%) at the occlusion time. No significant changes in serial echocardiography for two patients were found. All patients had taken anti-platelet and/or anticoagulant medicine after diagnosis. No death was recorded during follow-up.
TIMI grade 0 left coronary artery (LCA) occlusion was found in five patients (45.5%), LAD in four patients, and left circumflex coronary artery (LCX) in one patient; meanwhile, TIMI grade 0 RCA occlusion was found in ten patients (90.9%). Coronary artery bypass graft (CABG) was done for all patients with TIMI grade 0 LAD occlusion. Isolated RCA occlusion (TIMI grade 0) was found in six patients, and TIMI grade 0 RCA occlusion in one of two vessels was detected in two patients. TIMI grade 0 occlusion in two coronary arteries was found concurrently in two patients (LAD/RCA and LCX/RCA). Detailed descriptions of the patients with TIMI grade 0 LCA occlusion are listed below.
CAG at the time TIMI grade 0 occlusion was detected showed various degrees of collaterals (Rentrop grade 1-3), and the collaterals came from undefined other coronary arteries.
Among cases of isolated RCA total occlusion, most of the collaterals extended from the LCA and RCA proximal to occlusion. In patient 9, CAG of the RCA revealed TIMI grade 0 occlusion of RCA origin; there were no collaterals from the proximal RCA. In the other patients, some bridging vessels were found from the proximal RCA.
Among cases of LAD total occlusion, collaterals arose from the RCA and LCX. CAG of the LCA in patient 4 showed LAD total occlusion and grade 3 collaterals from the RCA, LCX, and proximal LCA, because the proximal LAD was preserved. We could not quantify the contribution of each patent coronary artery to collaterals.
Coronary intervention was performed in 6 patients, CABG in 4 patients, and percutaneous coronary intervention (PCI) in 2 patients of TIMI grade 1. The sites of intervention included the LAD and LCX. No intervention was performed for the RCA.
CABG was performed at the LAD in three TIMI grade 0 obstructions and one TIMI grade 1 stenosis. PCI was done at the LAD and LCX in cases of TIMI grade 1 stenosis and TIMI grade 0 obstruction. These showed good patency on follow-up CAG after CABG, but repeated interventions were necessary after PCI in two patients: CABG in one patient and a second PCI in the other.
In 4 patients, TIMI grade 0 RCA occlusion occurred after coronary intervention: three after CABG and one after PCI. The intervals from the time of CABG to RCA total occlusion were 6 months, 1 year, and 1.8 years. In cases of RCA occlusion after CABG to the LAD in three patients, a previous CAG showed that all RCAs had aneurysm or TIMI grade 1 stenosis (Fig. 1). Therefore, TIMI grade 0 RCA occlusion occurred in all cases that underwent CABG to the LAD except for one patient. This one patient underwent CABG to the LAD due to LAD total occlusion, whereas the LCX and RCA showed simultaneous aneurysm. Fortunately, RCA occlusion had not occurred till 4.5 years after CABG, and the stenosis progressed from TIMI grade 2 to TIMI grade 1 with each CAG. Accordingly, the next CAG was scheduled for close monitoring of the RCA. A new TIMI grade 0 RCA total occlusion presented in one patient initially treated with PCI for TIMI grade 1 LAD severe stenosis. This occurred four years after the initial PCI, and LAD total occlusion was also found on the same CAG at the age of 18.2 years. Another patient also underwent PCI after LCX and RCA total occlusion with TIMI grade 1 LAD stenosis were found on the first CAG. Percutaneous coronary intervention was performed three times for patency of the LAD and LCX. Thereafter, the patient was stable with patent LAD and LCX, although TIMI grade 0 RCA occlusion persisted.
Collaterals to the LAD in patients with total occlusion disappeared after CABG to the LAD on following CAG. However, interestingly, in a case of RCA total occlusion after LAD recanalization, prominent collaterals could be seen from the re-vascularized distal LAD (Fig. 1). There were some changes in the degree of collaterals to the RCA in three patients. The collaterals to the RCA occlusion after LAD recanalization decreased in two patients: Rentrop grade 3 decreased to Rentrop grade 2 on CAG 2.5 years later in one patient and Rentrop grade 2 to Rentrop grade 1 on CAG 3 years later in the other patient. Additionally, spontaneous decrease in collateral perfusion to the RCA on CAG after 1 year was found in one patient (from Rentrop gr. 2 to Rentrop gr. 1) (Fig. 2). Nevertheless, in spite of small changes in collateral perfusion, we did not observe any changes in cardiac manifestations.
Coronary artery stenosis in KD occurs frequently in patients with giant aneurysm and steadily progresses with time.2 However, severely stenotic or occlusive coronary arteries as a sequelae of KD develop collaterals in the chronic phase of KD, most of which develop sufficiently to prevent myocardial infarction.7 Therein, recanalization or collateral formation improves the perfusion to the distal myocardium of the occluded native coronary artery.8,12 Contrary to this, Tatara, et al.13 reported that the development of collateral vessels cannot protect against myocardial ischemia and the degree of collaterals have no effect on myocardial imaging. From our observations, only two patients out of eleven showed clinical symptoms associated with myocardial ischemia. This could be from the collaterals and protected myocardial viabilities, but the results from the SPECT showed perfusion defects in seven patients. Patient 6, who presented with LCX and RCA total occlusion, complained of chest pain, but SPECT showed normal perfusion. Some discrepant results were found in our clinical manifestations. It was mentioned that some of perfusion defects on thallium-201 SPECT could be possible due to extensive fibrosis as a sequel of acute myocarditis of KD.7 However, it has been reported that reversible perfusion defects can be seen in asymptomatic patients with KD.14 Kato, et al.15 reported that myocardial infarctions in KD were asymptomatic in 37% of patients. The reasons for asymptomatic myocardial ischemia in KD are not clear, but collateral circulation could ameliorate the degree of ischemia. For this reason, we performed regular CAG with SPECT, even in asymptomatic patients. A regular myocardial stress test and CAG should be done in patients with coronary arterial complications associated with KD. Fukuda, et al.12 proposed that unnecessary CAG could be postponed until the time at which ischemic findings change in the dipyridamole stress SPECT. With myocardial ischemia, there are no evidence-based guidelines for follow-up and evaluation of patients with coronary abnormalities after KD. Therefore, more well-designed studies are needed to determine the clinical implications of changes in collateral vessels on myocardial ischemia.
Of interest, we observed many RCA occlusions that occurred after LAD recanalization. Only one patient showed patent RCA after CABG to the LAD. The last CAG, performed at 4.5 years after CABG, also showed that RCA stenosis was becoming more severe. As we were not sure whether this RCA would keep its lifelong patency, this patient was decided to be followed up closely. The reason for RCA occlusion after LCA recanalization is unknown, but we propose that collaterals from recovered distal LCA flow could have provoked it. All cases of RCA occlusion occurred in less than 2 years after CABG. Therefore, we recommend regular follow-up CAGs until 2 years after CABG. Also, natural progression of RCA occlusion from severe stenosis could not be excluded because all RCAs had aneurysms or stenosis on a previous CAG. From our findings, changes in collaterals after LCA recanalization were natural, and we should expect significant changes in other coronary arteries after treatment. We could not explain the reasons for decrease in collaterals to the RCA in three patients. Nevertheless, there could be personal bias when the grade was analyzed. Coronary arterial remodeling in KD has not yet been clearly described,16 and to the best of our knowledge, very few reports on the assessment of the changes in collaterals that develop after occlusion of coronary arteries in KD have been published.12 The development of collateral vessels associated with total occlusion of coronary artery in infants and young children may contribute to a better prognosis,8 Some investigators have reported that myocardial viability is highly associated with the distribution of collateral blood flow within the occluded infarct bed.17 And obstructive lesions of the right coronary artery have been known to have a better prognosis than obstructive lesions of the left anterior descending coronary artery.8 A better understanding of the course of the coronary arterial remodeling in KD may lead to more innovative and effective treatment. In general, the guidelines for catheter intervention by the research committee of the Japanese Ministry of Health, Labor and Welfare have accepted it as a coronary intervention after KD.18 There are no clear indications for RCA intervention even in total occlusion. From our study, all patients with RCA occlusion had collaterals, and the patients did not have any symptoms, even those patients with decreased collaterals. Although RCA occlusion might be safe for myocardial ischemia, we found some changes in collaterals on follow-up CAG. Therefore, careful evaluation is important not only for the LAD and LCX, but also for the RCA. Especially, when considering CABG to the LCA, we should be careful of the possibility of RCA total occlusion after intervention. Close follow-up is recommended because of possible RCA occlusion of new onset. Further study regarding the development of myocardial perfusion in children is required to elucidate the process of change from localized stenosis to occlusion and the changes of collaterals.
There are several limitations in this study. First, this is a retrospective and descriptive study with a small number of patients, so we cannot expect our findings to be generalized to similar post-KD patients. Second, the follow-up duration was not enough for complete expectation. Third, the quantification of the collaterals was not specific for the coronary disease in KD. Finally, the grading system described by Rentrop lacks objectivity and could be biased.
In conclusion, even though clinical symptoms are subtle in coronary occlusion after KD, changes in collaterals and obstruction should be evaluated regularly for the prevention of further myocardial infarction. Regular evaluation for RCA might be needed as well, especially after CABG due to LAD occlusion.
Figures and Tables
Fig. 1
(Patient 4) Kawasaki disease was diagnosed at the age of 5 years and his first coronary angiography at the age of 5.9 years showed total occlusion of the LAD (A). RCA angiogram showed aneurysm and collaterals to the LAD (B). The next coronary angiography was performed 6 months after coronary artery bypass graft to the LAD and showed RCA total occlusion (C), collaterals from left circumflex coronary artery (arrow) (D), and CABG flow to left anterior descending coronary artery (arrow) (E). LAD, left anterior descending coronary artery; RCA, right coronary artery; CABG, coronary artery bypass graft.
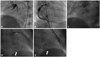
Fig. 2
(Patient 10) Kawasaki disease was diagnosed at the age of 3 months. His first coronary angiography was performed at the age of 1.5 years and showed right coronary artery total occlusion (A) with collaterals from left coronary artery (arrow) (B). Coronary angiography performed 1 year later demonstrated decrease in collaterals to the right coronary artery from the left coronary artery (C).

Table 1
Demographic Data and Clinical Manifestations at the Time of Total Occlusion of Coronary Artery
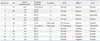
Table 2
The Serial CAG and the Results on Flow and Collaterals
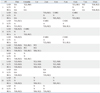
LAD, left anterior descending coronary artery; LCX, left circumflex coronary artery; RCA, right coronary artery; GA, giant aneurysm; N, normal; A, aneurysm; PCI, percutaneous coronary intervention; CABG, coronary artery bypass graft; T, thrombolysis in myocardial infarction; R, Rentrop; CAG, coronary angiographies.
References
1. Ortiz-Pérez JT, Rodríguez J, Meyers SN, Lee DC, Davidson C, Wu E. Correspondence between the 17-segment model and coronary arterial anatomy using contrast-enhanced cardiac magnetic resonance imaging. JACC Cardiovasc Imaging. 2008; 1:282–293.

2. Kato H, Sugimura T, Akagi T, Sato N, Hashino K, Maeno Y, et al. Long-term consequences of Kawasaki disease. A 10- to 21-year follow-up study of 594 patients. Circulation. 1996; 94:1379–1385.
3. Suzuki A, Kamiya T, Ono Y, Kinoshita Y, Kawamura S, Kimura K. Clinical significance of morphologic classification of coronary arterial segmental stenosis due to Kawasaki disease. Am J Cardiol. 1993; 71:1169–1173.

4. Shiraishi I, Onouchi Z, Hayano T, Hamaoka K, Kiyosawa N. Asymptomatic myocardial infarction in Kawasaki disease: long-term prognosis. Pediatr Cardiol. 1991; 12:78–82.

5. Tsuda E, Kitamura S. National survey of coronary artery bypass grafting for coronary stenosis caused by Kawasaki disease in Japan. Circulation. 2004; 110:11 Suppl 1. II61–II66.

6. Kitamura S, Tsuda E, Kobayashi J, Nakajima H, Yoshikawa Y, Yagihara T, et al. Twenty-five-year outcome of pediatric coronary artery bypass surgery for Kawasaki disease. Circulation. 2009; 120:60–68.

7. Onouchi Z, Hamaoka K, Kamiya Y, Hayashi S, Ohmochi Y, Sakata K, et al. Transformation of coronary artery aneurysm to obstructive lesion and the role of collateral vessels in myocardial perfusion in patients with Kawasaki disease. J Am Coll Cardiol. 1993; 21:158–162.

8. Kinoshita Y, Suzuki A, Nakajima T, Ono Y, Arakaki Y, Kamiya T. Collateral vessels assessed by myocardial contrast echocardiography in patients with coronary artery lesions after Kawasaki disease. Heart Vessels. 1996; 11:203–210.

9. TIMI Study Group. The Thrombolysis in Myocardial Infarction (TIMI) trial. Phase I findings. N Engl J Med. 1985; 312:932–936.
10. Gibson CM, Cannon CP, Daley WL, Dodge JT Jr, Alexander B Jr, Marble SJ, et al. TIMI frame count: a quantitative method of assessing coronary artery flow. Circulation. 1996; 93:879–888.
11. Cohen M, Rentrop KP. Limitation of myocardial ischemia by collateral circulation during sudden controlled coronary artery occlusion in human subjects: a prospective study. Circulation. 1986; 74:469–476.

12. Fukuda T, Ishibashi M, Shinohara T, Miyake T, Kudoh T, Saga T. Follow-up assessment of the collateral circulation in patients with Kawasaki disease who underwent dipyridamole stress technetium-99m tetrofosmin scintigraphy. Pediatr Cardiol. 2005; 26:558–564.

13. Tatara K, Murata M, Itoh K, Kazuma N, Kondo C. Management of severe coronary sequelae of Kawasaki disease. Am Heart J. 1996; 131:576–581.

14. Kashyap R, Mittal BR, Bhattacharya A, Manojkumar R, Singh S. Exercise myocardial perfusion imaging to evaluate inducible ischaemia in children with Kawasaki disease. Nucl Med Commun. 2011; 32:137–141.

15. Kato H, Ichinose E, Kawasaki T. Myocardial infarction in Kawasaki disease: clinical analyses in 195 cases. J Pediatr. 1986; 108:923–927.

16. Suzuki A, Miyagawa-Tomita S, Nakazawa M, Yutani C. Remodeling of coronary artery lesions due to Kawasaki disease: comparison of arteriographic and immunohistochemical findings. Jpn Heart J. 2000; 41:245–256.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download