Abstract
Purpose
Landmark indicators have not yet to be developed to detect the regression of cervical intraepithelial neoplasia (CIN). We propose that quantitative viral load and indicative histological criteria can be used to differentiate between atypical squamous cells of undetermined significance (ASCUS) and a CIN of grade 1.
Materials and Methods
We collected 115 tissue biopsies from women who tested positive for the human papilloma virus (HPV). Nine morphological parameters including nuclear size, perinuclear halo, hyperchromasia, typical koilocyte (TK), abortive koilocyte (AK), bi-/multi-nucleation, keratohyaline granules, inflammation, and dyskeratosis were examined for each case. Correlation analyses, cumulative logistic regression, and binary logistic regression were used to determine optimal cut-off values of HPV copy numbers. The parameters TK, perinuclear halo, multi-nucleation, and nuclear size were significantly correlated quantitatively to HPV copy number.
Because infection with the oncogenic human papilloma virus (HPV) represents one of the most important risk factors for initiation and progression of cervical carcinoma, HPV DNA detection tools have been developed with a triage algorithm not only to screen, but also to monitor cervical lesions. In comparison with the traditional Pap smear, HPV testing is now well known for its high sensitivity and excellent negative predictive value.1,2
The Hybrid Capture 2 (HC2) assay is a second-generation HPV DNA test that is especially useful for focusing on about two dozen major, high-risk types of HPV.1,3 It has the power to provide viral loading concentrations as a score to reflect viral burden in terms of replication or proliferation, although it is limited to detecting only high-risk groups.4 While even major genotypes of HPV infection are known to regress spontaneously,5 host and viral factors, including persistent or multiple infections, are significantly associated with progressive disease.6,7
As a candidate risk factor, the role of quantitative viral load has been debated to be preferentially associated with the histological severity of cervical intraepithelial neoplasia (CIN).5 In this study, we sought to correlate viral load numbers with the cytomorphological spectrum, including the vague regressing period of an HPV infection.
Traditionally, cervical cancer has been considered to be a multistep progression through CIN, from grades CIN1-3, and eventually to stromal invasion.8,9 However, since the clarification of HPV-infected koilocytosis, the "squamous intraepithelial lesion" (SIL) concept has been newly introduced to replace CIN. Thus, low-grade SIL is now regarded as a viral replicative stage and reversible lesion; whereas, high-grade SIL may be defined as a proliferative stage and a mostly irreversible lesion.10 The CIN2 group is very heterogeneous, including stages of both viral replication and cellular proliferation, as well as vaguely defined stages.11
Moreover, there is neither a clearly defined spectrum nor a specific pathological entity for cases in which HPV-infected cells, which are identified in CIN1, regress as a result of an immunological response. It is possible that the many cytological findings of atypical squamous cells of undetermined significance (ASCUS) could be compatible with stages of regressing koilocytosis.
Given an ASCUS-matched spectrum (from the Bethesda System), pathologists may recognize tissue findings with sufficient criteria so that ASCUS does not become a hodgepodge of ill-defined diagnostic categories. We attempted to determine findings from tissue biopsy specimens specific to cytological ASCUS and to clarify whether they are byproducts of an ongoing process of regressive findings following initial HPV infection.
Punch biopsy samples were collected at the Department of Pathology, Yonsei University College of Medicine, from January 2010 to July 2011. Individuals aged 21-55 years who were HPV-positive (HC2-positive) at the first study visit and positive more than three times over at least 2 consecutive years, responded to follow up at least three times, had no concomitant cancer, had no current referral for a hysterectomy during the subsequent 2 years, and had newly developed CIN1 or lower diagnosis by biopsy were included. Exclusion criteria were known CIN or cancer at baseline, a medical history of cervical pathology, HIV infection, or any immunosuppressive medication. Based on these criteria, we enrolled 115 biopsy samples from HPV-positive patients. All participants were monitored for high-risk HPV, using HC2 and Pap cytology smears for detecting cervical disease, at least three times during the subsequent 2 years. When cervical disease exceeding a low-grade SIL (LSIL) was detected during follow up, the disease was confirmed by histological examination.
The HC2 HPV test (Digene; Gaithersburg, MD, USA) was performed according to the manufacturer's instructions to detect HPV, using 4 mL of liquid cytology specimens. The HC2-13 high-risk probe cocktail detects HPV types 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, and 68.4 A relative light unit (RLU)/cut-off ratio between 1 and 2.5 RLU was used as the threshold for a positive result. The FDA-recommended cut-off value for a positive test result is 1.0 RLU (equivalent to 1 pg HPV DNA per 1 mL of sampling buffer). Digitized RLUs are known to be proportional to the amount of target DNA present in the specimen, thus providing a semiquantitative measure of viral load.
We designed a diagnostic algorithm using the following morphological variables in the selected, sharply demarcated lesions observed by low-magnification scanning: 1) nuclear enlargement to three times the size of adjacent intermediate cells; 2) bi- or multi-nucleation; 3) nuclear hyperchromasia; 4) perinuclear halo (pNcH); 5) typical koilocytes (TKs); 6) abortive koilocytes (AKs); 7) keratohyaline granules; 8) inflammatory cells interspersed in the epithelium; and 9) apoptotic dyskeratosis. For each variable, we examined the ratio of positive cells to all observed cells in the selected lesion (1, 4-6). Bi- or multi-nucleation (variable 2) was counted as an absolute number of applicable cells in the specific selected lesion. Nuclear hyperchromasia, keratohyaline granules, exocytotic inflammatory cells, and dyskeratosis were assessed as 3/2/1/0, according to the degree of change (with 0 representing no change; 3 representing maximum change). Each variable in our algorithm (% size, % pNcH, and % TK) used to determine a final score in a specific lesion, is defined as described below.
The enlarged cell refers to those with nuclear enlargement three times the size of adjacent intermediate cells.
TKs were defined when the following three conditions were satisfied: 1) size: nuclear enlargement at least three times the size of the adjacent intermediate cells; 2) hyperchromasia: grade 3; and 3) pNcH: presence of a definite clear zone surrounding the nucleus.
AKs were defined as superficial or intermediate cells that met only one or two of these conditions, showing some perinuclear cytoplasmic clearing, but lacking the large, sharply demarcated pNcH surrounded by a condensed cytoplasmic rim and nuclear atypia.12,13 The degree of AKs was coded according to the total number of AKs in the specific selected lesion as follows: 0, no AKs; 1, AK is ≥1 but <10; 2, AK is ≥10 but <20; 3, AK ≥20.
Nuclear chromatin was coded as either 1, nuclear chromatin similar to that of normal cells; 2, slightly increased chromatin; 3, definitely coarse chromatin. Bi- or multi-nucleation was counted as the total number of cells containing double- or multi-nucleated cells with regular chromatin pattern12 in the specific lesion. Keratohyaline granules were defined as basophilic granules mostly around the nucleus, resembling nuclear fragmentation,12 and they were coded as 0/1/2/3 according to the amount of granules in a specific lesion, with higher integers representing relatively greater numbers of granules. Inflammatory exocytosis was coded as 0/1/2/3 according to the number of lymphocytes within the epithelium of the specific lesion. Dyskeratosis was coded as 0/1/2/3 according to the number of cells showing dyskeratosis with normal-sized, regular, or spindle-shaped nuclei and deep orangeophilic cytoplasm in the specific lesion.12 Biopsy diagnoses were performed according to the World Health Organization classification and coded as follows: cervical epithelia within normal limits or chronic inflammation, 0; regressing koilocytosis focally composed of AKs or ASCUS, 1; CIN1, 2; CIN2, 3; CIN3, 4; and squamous cell carcinoma, 5. If a lesion showed a mixture of two different grades, we used the higher grade. All measurements were performed by two gynecologic pathologists in two consecutive rounds of assessment. The purpose of the first round was to teach and train both pathologists at the same time under a shared microscope. The second round was done as a double-blind examination. Cases with discrepancy were resolved by a third-round of review.
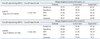
To assess the correlation between HPV copy number and morphological variables, correlation analyses were performed. Linear regression analysis was conducted between log (HPV) and the morphological scale values. Additionally, a cumulative logistic regression was performed between the biopsy diagnosis (0-5) and each morphological criterion with log (HPV). The biopsy scale was also analyzed according to focus using binary logistic regression on a narrow scale (0-2) or a wide scale (0-5). A correlative analysis was conducted using HPV load numbers and quantitative cytopathological scales based on different groupings of the scales [(0) and (1, 2)] versus [(0, 1) and (2)], to establish the dividing criteria between normal, subclinical lesions, and CIN1. Among the models derived from the regression analysis, the best-fit model for the calculation of cut-off values was selected based on the lowest Akaike information criterion (AIC) and the highest odds ratio (OR) (Table 1). Cut-off values were selected based on the priority of large area under the curve (AUC), and high positive and negative predictive values (PPV and NPV, respectively).
We performed a priori adjustment for age at each of the nine cytopathological scales and diagnoses based on cytology, focusing on subclinical LSIL (ASCUS) versus LSIL.
In practice, koilocytotic changes can be poorly defined and too broad even for experienced pathologists. Therefore, in the study observations from double-blinded microscopic examination of biopsy specimens were used to design a diagnostic algorithm covering the spectrum of koilocytotic changes to differentiate TKs from AKs. Here, TKs were defined using the following criteria: an acentric, hyperchromatic, and moderately enlarged nucleus that has been displaced by a large perinuclear halo. AKs cover a spectrum that did not meet all of the criteria of TKs; AKs were assessed according to the absolute number of AKs in five high-power fields of the selected lesion (Fig. 1). Reactive changes such as dyskeratosis, keratohyaline granules, and lymphocytes migrating into the epithelium were observed in up to 25%, 30%, and more than 50% of the cases, respectively.
Using our algorithm, we evaluated cut-off values in subclinical HPV lesions that were compatible with an ASCUS finding by cytopathology, as well as with CIN1 (LSIL with prominent koilocytosis) by tissue biopsy. Cut-off values based on the criteria of a large AUC, and high PPV or NPV are presented in Table 2.
Correlation analyses between HPV load number and the nine morphological criteria were performed. Morphological factors significantly correlated with the HPV quantitative copy number included the number of TKs (p<0.0001), degree of pNcH (p<0.0001), multi-nucleation (p=0.0193), and nuclear size (p=0.0315). The number of AKs was not correlated with HPV copy number.
We performed a multiple linear regression analysis of log (HPV) (dependent variable) against the nine morphological criteria (explanatory variables). Among the 12 models analyzed, model #12, with a combination of two major explanatory variables, i.e., the number of TKs and size (nuclear enlargement), showed the highest adjusted R2 value (0.3166; p<0.001) and was the best-fit model. The adjusted R2 value of 0.3166 (31.7%) indicated that 31.7% of the variability in log (HPV) could be explained by differences in TK number and size.
Cumulative logistic regression was performed to analyze the correlation between biopsy diagnosis scores (0-5; ordinal dependent variable) and the nine morphological criteria and log (HPV) (independent variable). Among the models analyzed, the best-fit model compatible with the lowest AIC (295.869) showed that factors correlating with an accurate diagnosis by tissue biopsy include log (HPV) (OR=1.183; p=0.0571), number of AKs (OR=0.629; p=0.0216), nuclear size (OR=1.041; p<0.0001), and age (OR=1.015; p=0.2971). These results indicate that higher HPV copy number, lower AKs frequency, greater nuclear size, and increased age result in a greater chance of HPV diagnosis by tissue biopsy. In all the models, number of TKs was the variable showing most significant correlation with HPV load (p<0.001).
Subsets of binary logistic regression were limited to biopsy diagnosis scores of 0-2 as the dependent variable. To determine the best-fit model, we selected the model with log (HPV) and AKs as independent variables showing the lowest AIC (92.227) and highest OR [log (HPV)=13.54] (Table 1) to calculate the cut-off values and receiver operating characteristic curves between biopsy scores [(0) and (1, 2)], and [(0, 1) and (2)]. In the selected model, HPV cut-off values of 58.9 and 271.49 were selected for biopsy scores [(0) and (1, 2)], and [(0, 1) and (2)], respectively, on the basis of maximizing AUC (Fig. 2). Fig. 3 summarizes the diagnostic algorithm using morphological scales in addition to the HC2 value. Load values of HPV above 58.9 and AK numbers above 20 were the optimal cut-off values to discriminate between negative findings (biopsy score 0) and equivocal changes (biopsy scores 1 and 2). Viral load above 271.49 and AK numbers >20 were the optimal cut-off values to discriminate between equivocal changes (biopsy scores 0 and 1) and obvious koilocytosis (biopsy score 2). Binary logistic regression, considering the wide range of the CIN spectrum as a dependent variable, was also performed. In cases where more than one-third of the cells showed increased size, an HPV load greater than 56.85 was the optimal cut-off value to discriminate between a subclinical diagnosis of undetermined significance (biopsy scores 0 and 1) and obvious clinical lesions (biopsy scores 2-5).
Commonly, HPV infects the nuclei of mitotically active basal cells in the epithelium, thus establishing an infective cycle. Spontaneous regression of HPV infection has been observed in 71.1% of one cohort.14 Patients who fail to clear the virus and remain persistently infected are at risk of progression to grade 2/3 CIN, the obligate precursor to invasive cervical cancer.15 It is known that HPV infection may result in latent, subclinical, and clinical infections.16 Latent infections have been difficult to examine because concentrations of viral DNA and RNA are low, and HPV may be harbored in cells/tissues subclinically at and beyond the apparently normal margins of many active, visible lesions, and in patients in remission.17
Replication of HPV in epithelial cells is accompanied by morphological cell alterations, resulting in pathogenic effects characteristic of HPV infection, allowing for morphological diagnosis of clinical HPV infections in Pap smears and biopsies with a high degree of accuracy.16 Koilocytes are squamous epithelial cells with an acentric, hyperchromatic nucleus that are displaced by a large pNcH as well as occasional multi-nucleation.18-20 However, given the evolving mechanisms and biology of HPV, cytopathological classification remains at a conventional stage and has failed to keep pace with the virology. Using only initially defined TKs, pathologists encounter a diagnostic conundrum. Over 70-90% of patients with HPV infection regress spontaneously. However, diagnostic criteria are too variable to assess and unify the changes in regression. This study was designed to clarify whether specific findings matching with ASCUS exist in subsequent biopsied tissues, and to determine if they are byproducts of the regressive process following initial HPV infection. To accomplish this, categorization of microscopic findings and a comparative tool based on reference values are essential prerequisites. Nine major findings and five groups of diagnoses with reference values of log (HPV) copy numbers were determined here.
As an additional challenge, numerous clinical cases include AKs that could also be classified as TK.21 AKs are frequently observed, but they are vaguely defined and seldom standardized. We suggest that the definition of AKs include the presence of superficial or intermediate cells meeting only one or two of the following criteria: 1) nuclear enlargement at three times the size of the adjacent intermediate cells; 2) hyperchromasia; and 3) pNcH. Whether the presence of HPV in AKs corresponds to an established infection is still debated.
In the present study, we found that HPV loading number (copy burden) significantly correlates with the number of TKs, degree of pNcH, multi-nucleation, and nuclear size. Because the number of AKs failed to show a correlation with HPV copy number, we suggest that cells indicative of AKs, whether early or late in the process, have lost the capacity to replicate virions. Moreover, well known supportive findings in characterizing koilocytes, including dyskeratosis and keratohyaline granules, do not correlate with HPV copy number. This indicates that both findings are not direct signs of active replication of virions, but rather residual effects of viral replication.
We found that a higher probability of an accurate biopsy diagnosis of an infective HPV lesion correlates with a greater HPV burden, lower AKs frequency, and larger nucleus. To date, quantitative HPV copy numbers (measured by the HC2 assay) have not been informative for pathologists or clinicians. Our findings suggest that viral load is informative in predicting whether HPV infection will follow a diminishing course or continue with active viral replication. Theoretically, HPV copy numbers should reach a plateau when viral replication is maximized in CIN1, and in the absence of further replication in higher grades of CIN, although the latter conclusion is controversial.8,22 In correlating HPV copy numbers with HPV fate following initial infection, we observed that viral load positively correlates with TK, size, pNcH, and multi-nucleation. In the present results, HPV burden numbers show significant correlation with pathological parameters, and from these we were able to predict the outcome of lesions very successfully. We were able to determine cut-off values to discriminate negative biopsy findings from ASCUS-compatible tissue findings, as well as to discriminate subclinical tissue findings from CIN1. Our results indicate that equivocal changes are likely when AKs numbers are greater than 20 and HPV copy numbers greater than 58.9. An HPV copy number greater than 271.49 with the same AKs findings might indicate CIN1.
A diagnostic algorithm combining HPV load with the number of AKs in biopsies could be useful to discriminate between sub-CIN1/regressing koilocytosis and CIN1. We developed such a diagnostic algorithm based on our findings here, and it is summarized in Fig. 3. Basic morphologic criteria for TK are nuclear size, pNcH, hyperchromasia, and/or multi-nucleation. If abnormal cells meet all of these criteria, we can diagnose the lesion as CIN1, confirming the HC2 positivity, and then plan to follow up after 3 months with HPV monitoring. On the other hand, if the cells do not meet any of the above criteria, we can diagnose the lesion as cervical epithelia within normal limits or chronic inflammation (CNI). For most of the cells in cervicovaginal swabs that are insufficient for a CIN1 diagnosis or equivocal identification of koilocytes, which could then be matched to ASCUS, we can divide these lesions into three categories according to criteria combining the number of AKs and HPV load (measured by HC2). When the lesion has more than 20 AKs and higher HC2 copy number than 271.49, we suggest it be regarded as CIN1; and when it has less than 20 AKs and HC2 number less than 58.9, we suggest it be treated as CNI. Finally, when the lesion has more than 20 AKs, with HPV copy numbers in the range of 58.9 to 271.49, we suggest it is reasonable to give these lesions an independent diagnosis of "regressing koilocytosis", in both the biological and pathological sense. The diagnostic term "regressing koilocytosis" can describe the infectious status of HPV, and it denotes the presumptive direction or fate of infection. Because it is based on the HPV loading number, this makes the lesional status clearer to communicate with clinicians for case management. To summarize, regressing koilocytosis can be narrowly defined as a squamous epithelial lesion, whether sharply or vaguely defined, with more than 20 AKs and with HPV copy numbers in the range of 58.9 to 271.49.
In conclusion, equivocal pathological findings characterized by AKs are compatible with ASCUS, or regressing koilocytosis, and they can be correlated with HPV load using optimal cut-off values.
Figures and Tables
Fig. 1
Microscopic examination of biopsied specimens: the spectrum of koilocytosis is variable between abortive and typical koilocytosis, from the diagnosis of CNI, cervical epithelia within normal limits or chronic inflammation (a), to CIN1, cervical intraepithelial neoplasm 1 (g), depending on whether the cell meets all the criteria of an acentric, hyperchromatic, moderately enlarged nucleus (c) displaced by a large perinuclear halo (f) or multinucleation (e). Other variables such as keratohyaline granules (b) and dyskeratosis, presumed to be inflammatory effects (d), were noted occasionally. AK, abortive koilocyte; TK, typical koilocyte.
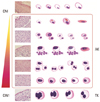
Fig. 2
Receiver operating characteristic (ROC) curves of log (HPV), AK, and a priori for age, for discrimination between (A) negative (0) and equivocal findings (1, 2); and (B) equivocal changes (0, 1) and obvious koilocytosis (2) in biopsies. AK, abortive koilocyte; HPV, human papilloma virus.

Fig. 3
Diagnostic algorithm for abnormal squamous cell lesions. Optimal cut-off values for ASCUS: regressing koilocytes matched ASCUS in the quantitative HPV copy number over the range of 58.9-271.49 when more than 20 AKs were detected.⊖, the cases which do not meet any of the above three criteria;⊕, the cases which meet only one of the above three criteria; , the cases which meet two of the above three criteria;
, the cases which meet two of the above three criteria; , the cases which meet all of the above three criteria. ASCUS, atypical squamous cells of undetermined significance; AK, abortive koilocyte; TK, typical koilocyte; HC, hybrid capture; CNI, cervical epithelia within normal limits or chronic inflammation; CIN1, cervical intraepithelial neoplasm 1; LR HPV, low risk human papilloma virus.
, the cases which meet all of the above three criteria. ASCUS, atypical squamous cells of undetermined significance; AK, abortive koilocyte; TK, typical koilocyte; HC, hybrid capture; CNI, cervical epithelia within normal limits or chronic inflammation; CIN1, cervical intraepithelial neoplasm 1; LR HPV, low risk human papilloma virus.
 , the cases which meet two of the above three criteria;
, the cases which meet two of the above three criteria; , the cases which meet all of the above three criteria. ASCUS, atypical squamous cells of undetermined significance; AK, abortive koilocyte; TK, typical koilocyte; HC, hybrid capture; CNI, cervical epithelia within normal limits or chronic inflammation; CIN1, cervical intraepithelial neoplasm 1; LR HPV, low risk human papilloma virus.
, the cases which meet all of the above three criteria. ASCUS, atypical squamous cells of undetermined significance; AK, abortive koilocyte; TK, typical koilocyte; HC, hybrid capture; CNI, cervical epithelia within normal limits or chronic inflammation; CIN1, cervical intraepithelial neoplasm 1; LR HPV, low risk human papilloma virus.
Table 1
Best-Fit Model Selection to Calculate Cut-Off Values between Biopsy Scores of [(0) and (1, 2)], and [(0, 1) and (2)], Based on the Lowest AIC and the Highest OR

ACKNOWLEDGEMENTS
This research was supported by the Mid-Career Researcher Program through the National Research Foundation of Korea grant, funded by the MEST (No. 2012R1A2A4A01006435; CNH), and by a faculty research grant from the Yonsei University College of Medicine (6-2012-0136; CNH). We also express our appreciation to Professor Ji Min Sung for help with statistical analyses, and Dong-su Jang for help in creating the illustrations used in the figures.
References
1. Kulmala SM, Syrjänen S, Shabalova I, Petrovichev N, Kozachenko V, Podistov J, et al. Human papillomavirus testing with the hybrid capture 2 assay and PCR as screening tools. J Clin Microbiol. 2004; 42:2470–2475.

2. Ratnam S, Franco EL, Ferenczy A. Human papillomavirus testing for primary screening of cervical cancer precursors. Cancer Epidemiol Biomarkers Prev. 2000; 9:945–951.
3. Venturoli S, Cricca M, Bonvicini F, Giosa F, Pulvirenti FR, Galli C, et al. Human papillomavirus DNA testing by PCR-ELISA and hybrid capture II from a single cytological specimen: concordance and correlation with cytological results. J Clin Virol. 2002; 25:177–185.

4. Tsiodras S, Georgoulakis J, Chranioti A, Voulgaris Z, Psyrri A, Tsivilika A, et al. Hybrid capture vs. PCR screening of cervical human papilloma virus infections. Cytological and histological associations in 1270 women. BMC Cancer. 2010; 10:53.

5. Clavel C, Masure M, Levert M, Putaud I, Mangeonjean C, Lorenzato M, et al. Human papillomavirus detection by the hybrid capture II assay: a reliable test to select women with normal cervical smears at risk for developing cervical lesions. Diagn Mol Pathol. 2000; 9:145–150.

6. Ylitalo N, Sørensen P, Josefsson AM, Magnusson PK, Andersen PK, Pontén J, et al. Consistent high viral load of human papillomavirus 16 and risk of cervical carcinoma in situ: a nested case-control study. Lancet. 2000; 355:2194–2198.

7. Ho GY, Bierman R, Beardsley L, Chang CJ, Burk RD. Natural history of cervicovaginal papillomavirus infection in young women. N Engl J Med. 1998; 338:423–428.

8. Tsai HT, Wu CH, Lai HL, Li RN, Tung YC, Chuang HY, et al. Association between quantitative high-risk human papillomavirus DNA load and cervical intraepithelial neoplasm risk. Cancer Epidemiol Biomarkers Prev. 2005; 14(11 Pt 1):2544–2549.

9. Knoepp SM, Kuebler DL, Wilbur DC. Correlation between hybrid capture II high-risk human papillomavirus DNA test chemiluminescence intensity from cervical samples with follow-up histologic results: a cytologic/histologic review of 367 cases. Cancer Cytopathol. 2010; 118:209–217.

10. Winer RL, Kiviat NB, Hughes JP, Adam DE, Lee SK, Kuypers JM, et al. Development and duration of human papillomavirus lesions, after initial infection. J Infect Dis. 2005; 191:731–738.

11. Wentzensen N, Wilson LE, Wheeler CM, Carreon JD, Gravitt PE, Schiffman M, et al. Hierarchical clustering of human papilloma virus genotype patterns in the ASCUS-LSIL triage study. Cancer Res. 2010; 70:8578–8586.

12. Bollmann M, Bánkfalvi A, Trosic A, Speich N, Schmittt C, Bollmann R. Can we detect cervical human papillomavirus (HPV) infection by cytomorphology alone? Diagnostic value of non-classic cytological signs of HPV effect in minimally abnormal Pap tests. Cytopathology. 2005; 16:13–21.

13. Nijhawan R, Mittal N, Suri V, Rajwanshi A. Enhancing the scope of conventional cervical cytology for detecting HPV infection. Diagn Cytopathol. 2010; 38:645–651.

14. An HJ, Sung JM, Park AR, Song KJ, Lee YN, Kim YT, et al. Prospective evaluation of longitudinal changes in human papillomavirus genotype and phylogenetic clade associated with cervical disease progression. Gynecol Oncol. 2011; 120:284–290.

15. Stanley M. Pathology and epidemiology of HPV infection in females. Gynecol Oncol. 2010; 117:2 Suppl. S5–S10.

16. Roteli-Martins CM, Alves VA, Santos RT, Martinez EZ, Syrjänen KJ, Derchain SF. Value of morphological criteria in diagnosing cervical HPV lesions confirmed by in situ hybridization and hybrid capture assay. Pathol Res Pract. 2001; 197:677–682.

17. Chow LT, Broker TR, Steinberg BM. The natural history of human papillomavirus infections of the mucosal epithelia. APMIS. 2010; 118:422–449.

18. Krawczyk E, Suprynowicz FA, Liu X, Dai Y, Hartmann DP, Hanover J, et al. Koilocytosis: a cooperative interaction between the human papillomavirus E5 and E6 oncoproteins. Am J Pathol. 2008; 173:682–688.
19. Moody CA, Laimins LA. Human papillomavirus oncoproteins: pathways to transformation. Nat Rev Cancer. 2010; 10:550–560.

20. Huh WK. Human papillomavirus infection: a concise review of natural history. Obstet Gynecol. 2009; 114:139–143.
21. Lawson JS, Glenn WK, Heng B, Ye Y, Tran B, Lutze-Mann L, et al. Koilocytes indicate a role for human papilloma virus in breast cancer. Br J Cancer. 2009; 101:1351–1356.

22. Sherman ME, Wang SS, Wheeler CM, Rich L, Gravitt PE, Tarone R, et al. Determinants of human papillomavirus load among women with histological cervical intraepithelial neoplasia 3: dominant impact of surrounding low-grade lesions. Cancer Epidemiol Biomarkers Prev. 2003; 12:1038–1044.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


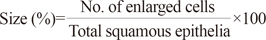
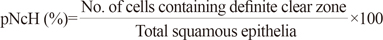
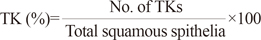
 XML Download
XML Download