Abstract
Purpose
Leuprorelin is a well known luteinizing hormone releasing hormone agonist. However, there are insufficient data on the efficacy and safety of high dose leuprorelin acetate, especially in Asian patients with prostate cancer. We aimed to investigate the safety and efficacy of leuprorelin acetate 22.5 mg administered at three-month intervals in patients with prostate cancer.
Materials and Methods
In an open, prospective clinical trial enrolling 47 patients, we aimed to assess the efficacy and safety of leuprorelin acetate 22.5 mg in treating patients with histologically confirmed prostate cancer. The primary objective of this study was to evaluate the efficacy of the leuprorelin acetate 22.5 mg in producing and maintaining castration levels of testosterone over a 6-month follow-up period and to determine its safety profile.
Results
All 42 patients achieved serum testosterone levels within the castration range by 4 weeks. A breakthrough response was observed in one of 36 patients by 8 weeks. However, this patient was medically castrated by 12 weeks. There were no significant prostate-specific antigen (PSA) or testosterone changes according to clinical stage or body mass index. Twenty adverse events (AEs) in 15 of 42 patients (35.7%) were observed during this study. The most common AEs were hot flushes (n=4, 20.0%) with mild intensity, pain (n=2, 10.0%), and infection (n=2, 10.0%). No patient withdrew from the study due to AEs.
In Korea, the incidence of prostate cancer has rapidly increased, and the increased incidence rate is highest among all forms of malignancy.1 With widespread prostate-specific antigen (PSA) screening, localized prostate cancer has exhibited the greatest increases in incidence rate among prostate cancer cases.2 PSA screening has facilitated discovery of a higher incidence of prostate cancer, as well as stage migration.3 From the CaPSURE database, despite early detection efforts and resulting stage migration in prostate cancer treatment, disease recurrence still develops in a significant proportion of patients after radiation therapy or radical prostatectomy.4
Androgen deprivation therapy (ADT) for prostate cancer was first introduced by Huggins and Hodges,5 and ADT remains one of the most effective palliative treatments for patients with prostate cancer. Luteinizing hormone-releasing hormone (LHRH) agonists act by down-regulating the pituitary gland, thereby suppressing secretion of luteinizing hormone and follicular stimulating hormone from the testis.6 Among several LHRH agonists, leuprorelin acetate 22.5 mg is a subcutaneous formulation designed to deliver 22.5 mg of leuprorelin acetate over 3 months and suppress serum testosterone levels over a 3-month period. However, there are insufficient data concerning the efficacy and safety of high dose leuprorelin acetate, especially in Asian patients with prostate cancer. Therefore, we aimed to investigate the safety and efficacy of leuprorelin acetate 22.5 mg administered at three-month intervals in patients with prostate cancer.
This multicenter (seven sites) study was designed to assess open-label, non-comparative administration of three monthly doses of leuprorelin acetate. The study protocol was approved by the institutional review boards of each center. Potential candidates provided written informed consent before participating in the screening.
Between August 2011 and May 2012, 47 patients who had histologically-confirmed prostate cancer were enrolled in this prospective study. Eligible patients included men with an Eastern Cooperative Oncology Group performance status of 0, 1, or 2, and no exposure to LHRH agonists, estrogens, anti-estrogens, antiandrogens, steroids or chemotherapeutic agents within 3 months of participating in the study. Exclusion criteria consisted of a history of penile, urethral, or scrotal surgery; current use of medications known to affect EF (e.g., phophodiesterase type 5 inhibitors); history of psychological disease, drug or alcohol dependence; men who suffered from any disease affecting testosterone levels; presence of another primary malignant neoplasm; men who suffered from known or suspected vertebral metastasis with risk of spinal compression; hypersensitivity to any of the study drugs or LHRH agonists; and history of participation in another clinical experiment within 3 months of the current study.
This study comprised a 2-week screening period and a 24-week therapeutic period. Patients who had histologically-confirmed prostate cancer and provided informed consent were screened for 2 weeks. Patients eligible for participation in the study were added to the intent-to-treat (ITT) populations.
During the therapeutic period, patients received two subcutaneous doses of leuprorelin acetate (Eligard® 22.5 mg HanAll Biopharmaceutical Co., Ltd., Seoul, Korea) at 12-week intervals for a total of 24 weeks. Efficacy and safety assessments were performed at 4, 8, 12, and 24 weeks, and quality of life (QOL), penile length, and testis volume were assessed before treatment and at each visit. In particular, measurement of penile length and testis volume were performed at three hospital sites, and the values for a total of 18 patients were assessed. The use of concomitant medications was monitored throughout the study period.
Efficacy was assessed by determining the percentage of patients who achieved serum testosterone levels below the castration range (≤50 ng/dL) at 4 weeks and maintained castration during the study period without a breakthrough response, which was defined as serum testosterone level >50 ng/dL, occurring after castration.
Safety assessments included regular examination for potential adverse events using the WHO classification; laboratory abnormalities including hematology, coagulation and blood chemistry at 4, 8, 12, and 24 weeks; and blood pressure. Patients were assessed before each injection unless otherwise stated. Investigators assessed the relationship between treatment and adverse events. Vital signs were also evaluated 2 and 4 hours after injection at day 1 and week 12.
QOL was evaluated before treatment and at 4, 8, 12, and 24 weeks using the Expanded Prostate Cancer Index (EPIC) sexual function domain.7 Patient self-assessment of bone pain, urinary pain, and urinary symptoms was determined throughout the study using a visual analog scale of 1 (no pain or no difficulty) to 10 (worst possible pain or very difficult). Penile length was measured by the same investigator with a paper ruler and was recorded to the nearest 0.5 cm. To avoid inter-observer variation, we used a spring scale to assure that each measurement was taken with a uniform stretching force (450 gm) using a technique previously described by Chen, et al.8 The measurements were conducted in a dimly lit and warm private room, with the patient in a supine position. Stretched penile length was measured from the pubo-penile skin junction to the tip of the glans, while applying perpendicular tension. Testis volume was measured by an ultrasound. The largest length, width and depth were measured, and volume was calculated using the formula V=/6×H×D×W.9
Data were analyzed using SPSS software (version 16.0, SPSS Inc., Chicago, IL, USA), with the efficacy analysis utilizing the ITT population, which included all randomized patients, regardless of protocol deviations, except for those who had missing testosterone values at 4 weeks. The safety analysis included all patients who received at least one dose of study medication, provided that they had safety data. Safety and QOL analyses were performed on the ITT population. The statistical comparisons consisted of post hoc analyses of the effects of baseline parameters on mean change in penile length (cm) and testis volume (cc) to each post-baseline assessment up to and including 24 weeks. Differences between penile length and testis volume measurements before and after treatment were evaluated using Bonferroni-corrected Wilcoxon's signed-rank tests. Statistical significance was defined as p<0.05 for all analyses.
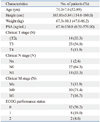
Patient demographics are shown in Table 1. Fourteen out of 42 patients had clinically localized prostate cancer, and 21 and 7 patients had locally advanced and metastatic prostate cancer.
All of the 42 patients achieved serum testosterone levels within the castration range by 4 weeks. A breakthrough response was observed in one of 36 patients by week 8; however, this patient was medically castrated by 12 weeks. Medical castration was achieved in all patients by 12 weeks and maintained up to 24 weeks. The mean PSA and testosterone changes after leuprorelin acetate treatment according to clinical stage are shown in Table 2. There were no significant PSA or testosterone changes according to clinical stage or body mass index.
No deaths were reported during this study. Twenty adverse events (AEs) in 15 patients among 42 patients (35.7%) were observed during this study. The most common AEs were hot flushes (n=4, 20.0%) of mild intensity, pain (n=2, 10.0%), and infection (n=2, 10.0%). No patient withdrew from the study due to AEs (Table 3).
Before treatment, the mean values for stretched penile length and testis volume (right and left) were 8.87±2.79 cm and 12.65±4.57 cc (right) and 16.52±23.17 cc (left), respectively. After ADT treatment, significant decreases in stretched penile length and testis volume were recorded: 8.14±2.83 cm and 7.07±2.67 cc (right) and 6.93±1.94 cc (left), respectively (p=0.046, 0.001). In regards to sexual function assessed on the EPIC questionnaire, statistically significant increases in erection problems were noted (p<0.01). Urinary difficulty via patient self-assessment was significantly reduced at 24 weeks compared with that at baseline (p<0.01, -1.07±1.48).
Eligard is a leuprorelin acetate formulation developed to increase the proportion of patients who achieve castration levels of testosterone and to reduce the occurrence of breakthrough responses, without increasing side effects. Eligard has twice the amount of leuprorelin found in other leuprorelin-based products and is available in three doses: 7.5 mg (0.25 mL), 22.5 mg (0.375 mL), and 45 mg (0.375 mL).10 In a previous clinical trial,11 Eligard was shown to be highly effective in reducing mean testosterone levels to below castration levels established by the FDA (≤50 ng/dL). Breakthrough was also infrequent and transient compared to our study. However, Heyns, et al.12 reported that repeated exposure to a higher dose was more likely to cause an escape as a result of weak desensitization of pituitary GnRH receptors. These contradictory results might be related to racial disparities. Further studies should address the mechanisms underlying the differences in the clinical responses to ADT between Asian people and Caucasian people.
Our study revealed that castration levels of testosterone could be achieved after treatment with leuprorelin acetate, regardless of baseline testosterone or clinical stage. The mean baseline testosterone level was 3262 ng/dL in 14 localized prostate cancer patients, and it decreased to 13.6 ng/dL at 4 weeks after treatment with leuprorelin acetate. Nevertheless, another study reported that a higher baseline testosterone level delays the achievement of castration testosterone levels with leuprorelin.13 An additional report suggested that patients with higher baseline testosterone levels receiving a GnRH agonist may be at increased risk of tumor stimulation and clinical flare.14 Notwithstanding, the present results suggest that a high dose of leuprorelin may induce castration more rapidly than other GnRH agonists, possibly due to differences in dose.
Reportedly, greater BMI is associated with higher serum levels of total testosterone during GnRH agonist treatment than normal BMI.15 Obese men had total and free testosterone levels 1.8-fold and 2.3-fold greater than normal men after 48 weeks of GnRH agonist treatment, respectively. Other investigators have reported failure to maintain castration testosterone levels during GnRH agonist therapy.16,17 However, in our study showed, obese patients (BMI >25 kg/m2) achieved serum testosterone levels within the castration range by 24 weeks, contradictory to previous results. This result may be due to ethnic differences of lower BMI in Asians compared with Caucasians. Future studies are needed to evaluate the relationship between sex steroid levels and survival during treatment with a GnRH agonist and to determine whether interventions to further decrease sex steroids levels improve clinical outcomes.
The high-dose LHRH agonist used in this study has been widely utilized in Western countries for 10 years, but is not yet utilized with Asian populations. According to a previous study,18 Asian people had higher testosterone levels, but a lower ratio of dihydrotestosterone to testosterone than African-American or Caucasian men. In addition, Asian people have a longer amino-terminal trinucleotide repeat length of the androgen receptor gene, which is associated with the incidence and severity of prostate cancer.19 However, in the present study, leuprorelin acetate at 22.5 mg effectively suppressed serum testosterone levels despite racial differences, in comparison to other studies.11,20 Therefore, racial differences in sex hormone levels and the aggressive biology of prostate cancer in Korean people do not seem to adversely affect responses to ADT.
In our study, the most common AE was hot flushes (n=4, 20.0%). The incidences of AEs were relatively lower compared to those reported in another study,19 even though our study was conducted with a double-dosed LHRH agonist. These results demonstrate that high-dose leuprorelin acetate did not induce higher AEs compared with low-dose leuprorelin acetate.
This prospective study had some limitations. Our study had a relatively short follow-up period and small sample size. In addition, we did not assess tumor responses after ADT treatment because of the difficulty of assessing this endpoint in men with PCa. Furthermore, future studies are needed to clarify the relationship between the extent of testosterone suppression, time to cancer progression, and cancer-specific survival.
In the present study, medical castration was achieved in all patients by 12 weeks and maintained up to 24 weeks. Leuprorelin acetate 22.5 mg was shown to be effective and safe in Asian patients with prostate cancer, even though sexual function decreased during the study. Further long term follow up study is needed to confirm our results.
Figures and Tables
ACKNOWLEDGEMENTS
This study was supported by grants and drugs from HanAll Biopharmaceutical Co., Ltd., Seoul, South Korea.
References
1. Statistics Korea. accessed on February 5, 2013. Available at: http://kostat.go.kr/portal/english/index.action.
2. Lee DH, Jung HB, Chung MS, Lee SH, Chung BH. The change of prostate cancer treatment in Korea: 5 year analysis of a single institution. Yonsei Med J. 2013; 54:87–91.

4. Agarwal PK, Sadetsky N, Konety BR, Resnick MI, Carroll PR. Treatment failure after primary and salvage therapy for prostate cancer: likelihood, patterns of care, and outcomes. Cancer. 2008; 112:307–314.

5. Huggins C, Hodges CV. Studies on prostatic cancer: I. The effect of castration, of estrogen and of androgen injection on serum phosphatases in metastatic carcinoma of the prostate. 1941. J Urol. 2002; 168:9–12.

6. Tolis G, Ackman D, Stellos A, Mehta A, Labrie F, Fazekas AT, et al. Tumor growth inhibition in patients with prostatic carcinoma treated with luteinizing hormone-releasing hormone agonists. Proc Natl Acad Sci U S A. 1982; 79:1658–1662.

7. Chung KJ, Kim JJ, Lim SH, Kim TH, Han DH, Lee SW. Development and validation of the Korean version of expanded prostate cancer index composite: questionnaire assessing health-related quality of life after prostate cancer treatment. Korean J Urol. 2010; 51:601–612.

8. Chen J, Gefen A, Greenstein A, Matzkin H, Elad D. Predicting penile size during erection. Int J Impot Res. 2000; 12:328–333.

9. Chipkevitch E, Nishimura RT, Tu DG, Galea-Rojas M. Clinical measurement of testicular volume in adolescents: comparison of the reliability of 5 methods. J Urol. 1996; 156:2050–2053.

10. Berges R, Bello U. Effect of a new leuprorelin formulation on testosterone levels in patients with advanced prostate cancer. Curr Med Res Opin. 2006; 22:649–655.

11. Chu FM, Jayson M, Dineen MK, Perez R, Harkaway R, Tyler RC. A clinical study of 22.5 mg. La-2550: a new subcutaneous depot delivery system for leuprolide acetate for the treatment of prostate cancer. J Urol. 2002; 168:1199–1203.

12. Heyns CF, Simonin MP, Grosgurin P, Schall R, Porchet HC. South African Triptorelin Study Group. Comparative efficacy of triptorelin pamoate and leuprolide acetate in men with advanced prostate cancer. BJU Int. 2003; 92:226–231.

13. Damber JE, Tammela TL, Iversen P, Abrahamsson PA, Boccon-Gibod L, Olesen TK, et al. The effect of baseline testosterone on the efficacy of degarelix and leuprolide: further insights from a 12-month, comparative, phase III study in prostate cancer patients. Urology. 2012; 80:174–180.

14. Zagars GK, Pollack A, von Eschenbach AC. Serum testosterone--a significant determinant of metastatic relapse for irradiated localized prostate cancer. Urology. 1997; 49:327–334.

15. Smith MR. Obesity and sex steroids during gonadotropin-releasing hormone agonist treatment for prostate cancer. Clin Cancer Res. 2007; 13:241–245.

16. Oefelein MG, Cornum R. Failure to achieve castrate levels of testosterone during luteinizing hormone releasing hormone agonist therapy: the case for monitoring serum testosterone and a treatment decision algorithm. J Urol. 2000; 164(3 Pt 1):726–729.

17. Morote J, Esquena S, Abascal JM, Trilla E, Cecchini L, Raventós CX, et al. Failure to maintain a suppressed level of serum testosterone during long-acting depot luteinizing hormone-releasing hormone agonist therapy in patients with advanced prostate cancer. Urol Int. 2006; 77:135–138.

18. Wu AH, Whittemore AS, Kolonel LN, John EM, Gallagher RP, West DW, et al. Serum androgens and sex hormone-binding globulins in relation to lifestyle factors in older African-American, white, and Asian men in the United States and Canada. Cancer Epidemiol Biomarkers Prev. 1995; 4:735–741.
19. Pettaway CA. Racial differences in the androgen/androgen receptor pathway in prostate cancer. J Natl Med Assoc. 1999; 91:653–660.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download