Abstract
To date, the use of red blood cells (RBCs) produced from stem cells in vitro has not proved practical for routine transfusion. However, the perpetual and widespread shortage of blood products, problems related to transfusion-transmitted infections, and new emerging pathogens elicit an increasing demand for artificial blood. Worldwide efforts to achieve the goal of RBC production through stem cell research have received vast attention; however, problems with large-scale production and cost effectiveness have yet to prove practical usefulness. Some progress has been made, though, as cord blood stem cells and embryonic stem cells have shown an ability to differentiate and proliferate, and induced pluripotent stem cells have been shown to be an unlimited source for RBC production. However, transfusion of stem cell-derived RBCs still presents a number of challenges to overcome. This paper will summarize an up to date account of research and advances in stem cell-derived RBCs, delineate our laboratory protocol in producing RBCs from cord blood, and introduce the technological developments and limitations to current RBC production practices.
Transfusion of red blood cells (RBCs) is a standard and indispensable therapy for anemic conditions in current clinical practice. RBCs, comprised of hemoglobin and iron, are vital to sustaining life and functions to carry oxygen to cells and to transport CO2 out of the body through the lungs. Throughout history, the practice of blood transfusions progressed through many developments. During World War I, when many trauma related hemorrhages were prevalent, the necessity of transfusions brought about the identification of ABO groupings. During World War II, acid-citrate-dextrose blood preservation solution was developed.1 Later, in 1990, the ABO gene was cloned and sequenced by Yamamoto, et al.2 However, despite its importance, blood transfusion has been challenged by infectious transmissions with variant Creutzfeldt-Jakob disease, West Nile Virus, H1N1 virus, hepatitis, HIV, and other emerging pathogens, which continually threaten patient safety. Additionally, the growing demand of transfusions in modern medicine has caused supply limitations, which cannot be met by blood donations alone.3,4
Current practices that successfully treat chronic anemic conditions include recombinant erythropoietin stimulating agent therapy. In normal hematopoiesis, stem cell differentiation and maturation to RBCs require erythropoietin, which is the most important growth factor. In states of low erythropoietin production as in chronic kidney disease, recombinant erythropoietin stimulating agent therapy has been crucial in managing anemia. However, thrombotic and neoplastic risks limit its use in many cases.5 In more acute and emergent settings such as hemorrhagic conditions and post surgical states, blood transfusion is currently the only option. Also, in cases of rare blood phenotypes such as the universal type O/D- or allo-immunized recipients, transfusion is difficult to achieve.
Due to these challenges, RBC production from hematopoietic stem cells has been a focus in regenerative medicine.6-8 Undifferentiated stem cells confer the advantage of lifelong production in bone marrow. Given the right conditions, these stem cells have the potential to differentiate into mature RBCs after undergoing multiple steps in maturation. Despite the novelty of this recent tactic, many studies have been optimistic toward this end, and clinical therapeutic use may be possible sooner than previously predicted. If successful, this may be a solution to the worldwide shortage of blood supply. Our paper aims to discuss the prospects of the production of RBCs from stem cells based on our research experience.9-12
Hematopoietic stem cell-derived blood cells undergo a number of differentiation steps prior to proliferation. Stem cells originate in early stages of embyronic fertilization in the yolk sac for 2 months and in the liver and spleen during the 6th week of fetal development to 2 weeks after birth. From 6-7 months following birth, hematopoiesis continues in the bone marrow through childhood and then throughout adulthood. During development, the blood producing totipotent stem cells have self regenerating ability, which allows for continued red cell production and differentiation, which then is regulated by negative feedback to control the number of RBCs in the body. Robust bone marrow is able to produce a daily RBC count of 2.5 billions/kg, platelets of 7 millions/kg, and granulocytes of 850,000/kg. To differentiate into mature cells, RBCs require 5 days, platelets 7 days, and granulocytes 5-7 days.13 Depending on the cell type needed in the body, the hematopoietic stem cell factors influence differentiation into distinct lineages to produce stromal cells, endothelial progenitor cells, lymphocytes, fibroblasts, and macrophages. The earliest RBC progenitor is the burst forming unit erythroid, which then differentiates into proerythroblast cells, which are dependent on erythopoietin to prevent apoptosis. The availability of erythopoietin, which is produced in the kidney in response to hypoxic stimulation, regulates the amount of blood cell production. The hemoglobin synthesis pathway continues by a step-wise reduction of cell size, differentiating from the proerythroblast phase to basophilic normoblasts to polychromatic normoblasts to orthochromatic normoblasts to reticulocytes and finally to mature RBCs. Enucleation occurs immediately before the formation of the reticulocyte phase, which is thought to occur through macrophage contact with erythrocyte precursor. In addition to erythropoietin, 20 or more recombinant growth factors have been used for laboratory induction of bone marrow-derived cell differentiation including thrombopoietin and granulocyte colony stimulating factor (G-CSF). Cytokines such as stem cell factor (SCF) and vascular endothelial growth factor encourage promotion of cell production, while IL-6, TGF-beta, and INF-γ function to provide negative feedback to limit overproduction.14
RBC production from hematopoietic stem cells begins with the use of CD34 marker, a glycoprotein found in the bone marrow and expressed on early hematopoietic stem cells. CD34+ cells can also be found in cord blood and small amounts of G-CSF mobilized peripheral blood stem cell concentrates in the peripheral blood.15 By using CD34+ cells from cord blood and peripheral blood, Giarratana, et al.6,7,16 showed that the hematopoietic process can be reproduced, to an extent, in vivo. In this study, 10 million RBCs (the equivalent of 2 mL of blood) used for the first-in-man administration were derived from autologous CD34+ cells mobilized by G-CSF.16 However, problems related to enucleation, mass production, and high financial costs failed to produce conclusive evidence for practical use, warranting further studies. Following Giarratana's publications, in vitro production of red blood cells has been attempted by various researchers. Nevertheless, each laboratory utilized similar but different amounts and types of cytokines, culture media, and supplement concentrates.17-19
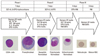
In this section, we discuss the methods used in our laboratory.10,12 From cord blood CD34+ cells, the maturation process of RBCs was divided into four steps (Fig. 1). In the first step, within 6 hours of post cord blood collection, mononuclear cells were separated using the Ficoll-Hypaque technique (density, 1.077; Pharmacia Biotech, Uppsala, Sweden) and subsequently using the MACS cell separation system (Miltenyi Biotech, Auburn, CA, USA) to isolate CD34+ cells. CD34+ cells were initially separated by MediMACS and then again by MiniMACS to achieve over 95% isolation purity. Sorted CD34+ cells were seeded in 24-well plates at a concentration of 1×105 cells per well. CD34+ cells were then continually cultured for the first 7 days in serum-free conditioned erythrocyte culture medium (StemPro-34 SFM Complete Medium, Gibco, Grad Island, NY, USA) with three kinds of cytokines, SCF (Peprotech, Rehovo, Israel) 100 ng/mL, IL-3 (Peprotech) 10 ng/mL, and recombinant erythropoietin (EPO, Recormon Epoetin beta, Roche, Mannheim, Germany) 6 IU/mL, with a half-medium change twice a week. In the second step, serum free conditioned medium with 3 IU/mL of recombinant EPO, 50 ng/mL of SCF, and 10 ng/mL of IL-3 were used for expansion and differentiation for 7 days. In the third step, 4 days of culture of only one cytokine (EPO 2 IU/mL) was used for erythrocyte differentiation, and 0.05% of poloxamer 188 [Pluronic F68 (F68), Sigma Chemical Co., St Louis, MO, USA; MW 8400], which is a nonionic block copolymer chemical surfactant known to be cytoprotective against hydrodynamic stress, was added. In the fourth step, 3 days of culture was performed with only poloxamer 188 without any cytokines in the final culture medium. All cultures were maintained at 37℃ in a humidified atmosphere of 5% CO2. In general, enucleation began after the 10th day, and mature cells were found starting day 17. Inexplicably, not all CD34+ cells achieved maturation (Fig. 2). However, previous studies showed that factors influencing the success of enucleation are correlated with the proper EPO concentration, compact optimal confluences of cells, and appropriate culture conditions mimicking the bone marrow stromal cell microenvironment milieu.20,21
Since these promising studies have begun in 2005, several industries and governments have invested in large scale blood manufacturing. For example, in 2009, the Defense Advanced Research Projects Agency (DARPA) in the USA began the "Blood Pharming" program, which aimed to provide a self-contained, synthetic platform for cord blood stem cell-derived RBCs in scale and quality that can meet the tremendous demands of the battlefield.22 DARPA collaborated with Arteriocyte Inc. (Cleveland, OH, USA), a biotech company, to develop novel technologies for in vitro production of RBCs that are untainted, readily available, and free of storage lesions. The ultimate target of the program was the development of an automated, fieldable cell culture, and packaging system capable of producing transfusable amounts of universal donor RBCs using human progenitor cells as starting material. Arteriocyte was awarded nearly $2 million to develop this genetically engineered blood product and made the first shipment to the FDA in 2011, hoping that the regulators will approve it for use in nationwide trauma wards. They showed the ability to turn one unit of umbilical cord blood into 20 units of blood in about 3 days at a cost of about $5,000 per unit. The cost is somewhat steep; nevertheless, if the FDA approves the blood product and Arteriocyte can bring down the cost and scale the production method, pharmed blood is projected to replace donated blood within five years. The use thereof was expected to begin in 2013; however, it has not yet been made available. In addition, hemoglobin-based oxygen carriers and perfluorocarbon emulsions have been studied as acellular artificial blood substitutes, although they have not been clinically available for use.23 Hematopoietic induction of stem cells and artificial blood production have been studied worldwide including the USA, England, Japan, and Australia.15-19,24 When considering these options, the safety and cost effectiveness thereof are paramount. We feel that these issues can be overcome in the near future to replenish much needed supplies of RBCs.
In 1993, erythroid colonies were separated from murine ESCs by Keller, et al.25 In their study, the embryonic body was disaggregated, and hematopoiesis was attempted in the setting of erythropoietin and c kit ligand containing medium. Although the primitive erythroid cells were derived, mature RBCs were not produced. In another lab, human embryonic stem cells were first cloned in 1998.26 As well, in the same lab, human hematopoietic colony formation and mature RBC formation from human ESCs replicated results from murine models, although complete success in full RBC maturation was again unable to be seen.27 Despite this, these studies provided much information regarding the erythropoietic process, which was helpful in molecular studies, such as in defining the importance of transcription factor functions in erythropoiesis.
Several groups showed that erythroblasts could be generated from ESCs, but the RBC yield was very low.28,29 In an industrial trial, Advanced Cell Technology (Worcester, MA, USA) first reported that ESCs differentiated into functional oxygen-carrying erythrocytes on a large scale (1010~1011 cells/6-well plate hESCs) as a source for clinical grade mass production of RBCs from stem cells.30 However, final RBC products from ESCs must be thoroughly evaluated for enucleation and overall safety. Meanwhile, such research faces further challenges, as ethical questions have arisen in regards to the procurement of embryonic stem cells and studies have been halted.
In 2010, induced pluripotent stem cells (iPSCs) were studied for therapeutic use, which eliminated the controversial use of embryos. The derivation of RBCs from iPSCs was first reported,31 and using the model of hematopoieisis, cultured RBCs from sickle-cell disease patients and normal human adult-iPSCs achieved terminal maturation in vitro in terms of enuclation. Furthermore, the establishment of immortalized human erythroid progenitor cells lines from human iPSCs could be a valuable model system to study both in vitro and in vivo differentiation processes in genetic disorders of erythropoiesis. Peyrard, et al.32 suggested that 15 iPSC lines representing the most useful RBC phenotypes would be sufficient to manage 100% of allo-immunized patient. Unfortunately, current methods for the production of hESC/iPSCs are extremely inefficient and costly. However, the need for an unlimited source and production of a "universal donor" RBC product will undoubtedly demand continuous research via the ESC/iPSC derived RBC production project.
In the 21st century, regenerative medicine has been introduced as a novel therapy that has resulted in a paradigm shift in the medical arena. However, for these therapeutic methods to be utilized in clinical settings, another 10-15 years of research is anticipated.33 Use of somatic cells and ESCs, as well as cord blood-induced hematopoiesis research is well under way. Limitations of current research include high costs, as with iPSCs. In the case of artificial RBCs, progress has not been made on a large enough scale to justify substitution of current methods of transfusion. In the case of CD34+ stem cell differentiation, research is limited to the laboratory at this time, and mass production is not yet possible, precluding availability for use. Engineering technology such as bioreactor use may be necessary for successful advances.
Therefore, we discerned that despite many advances in stem cell-derived hematopoiesis research, we are still in the beginning stages of making RBCs available, in both quantity and quality, for clinical use. Bone marrow-derived stem cell differentiation of RBCs seems to be a physiologic and natural way of RBC procurement and would be an attractive option if functionality and production quantity can be optimized. As the longevity of our population has increased and medical care is often reliant on adequate RBC management, further research is paramount. Although a number of challenges must be overcome before the clinical usage of stem cell-derived RBCs, we are optimistic about the prospects of it becoming a reality.
Figures and Tables
ACKNOWLEDGEMENTS
This project was supported by a grant from the Korean Healthcare Technology R&D Project, Ministry of Health and Welfare, Republic of Korea (HI10C1740).
References
2. Yamamoto F, Marken J, Tsuji T, White T, Clausen H, Hakomori S. Cloning and characterization of DNA complementary to human UDP-GalNAc: Fuc alpha 1----2Gal alpha 1----3GalNAc transferase (histo-blood group A transferase) mRNA. J Biol Chem. 1990; 265:1146–1151.

3. Ali A, Auvinen MK, Rautonen J. The aging population poses a global challenge for blood services. Transfusion. 2010; 50:584–588.
4. Seifried E, Klueter H, Weidmann C, Staudenmaier T, Schrezenmeier H, Henschler R, et al. How much blood is needed? Vox Sang. 2011; 100:10–21.

5. Buemi M, Lacquaniti A, Bolignano D, Cernaro V, Campo S, Grasso G, et al. Down with the erythropoietin. Long live the erythropoietin! Curr Drug Targets. 2009; 10:1028–1032.
6. Neildez-Nguyen TM, Wajcman H, Marden MC, Bensidhoum M, Moncollin V, Giarratana MC, et al. Human erythroid cells produced ex vivo at large scale differentiate into red blood cells in vivo. Nat Biotechnol. 2002; 20:467–472.

7. Giarratana MC, Kobari L, Lapillonne H, Chalmers D, Kiger L, Cynober T, et al. Ex vivo generation of fully mature human red blood cells from hematopoietic stem cells. Nat Biotechnol. 2005; 23:69–74.

8. Miharada K, Hiroyama T, Sudo K, Nagasawa T, Nakamura Y. Efficient enucleation of erythroblasts differentiated in vitro from hematopoietic stem and progenitor cells. Nat Biotechnol. 2006; 24:1255–1256.

9. Baek EJ, Kim HS, Kim S, Jin H, Choi TY, Kim HO. In vitro clinical-grade generation of red blood cells from human umbilical cord blood CD34+ cells. Transfusion. 2008; 48:2235–2245.

10. Baek EJ, Kim HS, Kim JH, Kim NJ, Kim HO. Stroma-free mass production of clinical-grade red blood cells (RBCs) by using poloxamer 188 as an RBC survival enhancer. Transfusion. 2009; 49:2285–2295.

11. Baek EJ, You J, Kim MS, Lee SY, Cho SJ, Kim E, et al. Enhanced production of red blood cells in suspension by electrostatic interactions with culture plates. Tissue Eng Part C Methods. 2010; 16:1325–1334.

12. Kim HO, Baek EJ. Red blood cell engineering in stroma and serum/plasma-free conditions and long term storage. Tissue Eng Part A. 2012; 18:117–126.

13. Koury MJ, Lichtman MA. Structure of the marrow and the hematopoietic microenvironment. In : Kaushansky K, Williams WJ, editors. Williams hematology. 8th ed. New York: McGraw-Hill Medical;2010. p. 41–73.
14. Tsiftsoglou AS, Vizirianakis IS, Strouboulis J. Erythropoiesis: model systems, molecular regulators, and developmental programs. IUBMB Life. 2009; 61:800–830.

15. Boehm D, Murphy WG, Al-Rubeai M. The potential of human peripheral blood derived CD34+ cells for ex vivo red blood cell production. J Biotechnol. 2009; 144:127–134.

16. Giarratana MC, Rouard H, Dumont A, Kiger L, Safeukui I, Le Pennec PY, et al. Proof of principle for transfusion of in vitro-generated red blood cells. Blood. 2011; 118:5071–5079.

17. Douay L, Giarratana MC. Ex vivo generation of human red blood cells: a new advance in stem cell engineering. Methods Mol Biol. 2009; 482:127–140.

18. Mountford J, Olivier E, Turner M. Prospects for the manufacture of red cells for transfusion. Br J Haematol. 2010; 149:22–34.

19. Nakamura Y. In vitro production of transfusable red blood cells. Biotechnol Genet Eng Rev. 2008; 25:187–201.

20. Choi HS, Lee EM, Kim HO, Park MI, Baek EJ. Autonomous control of terminal erythropoiesis via physical interactions among erythroid cells. Stem Cell Res. 2013; 10:442–453.

21. Park KS, Ahn J, Kim JY, Park H, Kim HO, Lee SH. Poly-l-lysine increases the ex vivo expansion and erythroid differentiation of human hematopoietic stem cells, as well as erythroid enucleation efficacy. Tissue Eng Part A. 2014; 01. 24. [Epub ahead of print].
22. Defense Advanced Research Projects Agency. Defense Sciences Office. Available at: http://www.darpa.mil/Our_Work/DSO/Programs/Blood_Pharming.aspx.
23. DeWitt JC, Peden-Adams MM, Keller JM, Germolec DR. Immunotoxicity of perfluorinated compounds: recent developments. Toxicol Pathol. 2012; 40:300–311.

24. Timmins NE, Nielsen LK. Blood cell manufacture: current methods and future challenges. Trends Biotechnol. 2009; 27:415–422.

25. Keller G, Kennedy M, Papayannopoulou T, Wiles MV. Hematopoietic commitment during embryonic stem cell differentiation in culture. Mol Cell Biol. 1993; 13:473–486.

26. Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998; 282:1145–1147.

27. Kaufman DS, Hanson ET, Lewis RL, Auerbach R, Thomson JA. Hematopoietic colony-forming cells derived from human embryonic stem cells. Proc Natl Acad Sci U S A. 2001; 98:10716–10721.

28. Ma F, Ebihara Y, Umeda K, Sakai H, Hanada S, Zhang H, et al. Generation of functional erythrocytes from human embryonic stem cell-derived definitive hematopoiesis. Proc Natl Acad Sci U S A. 2008; 105:13087–13092.

29. Mountford JC, Olivier E, Jordanides NE, de Sousa P, Turner ML. Red blood cells from pluripotent stem cells for use in transfusion. Regen Med. 2010; 5:411–423.

30. Lu SJ, Feng Q, Park JS, Vida L, Lee BS, Strausbauch M, et al. Biologic properties and enucleation of red blood cells from human embryonic stem cells. Blood. 2008; 112:4475–4484.

31. Lapillonne H, Kobari L, Mazurier C, Tropel P, Giarratana MC, Zanella-Cleon I, et al. Red blood cell generation from human induced pluripotent stem cells: perspectives for transfusion medicine. Haematologica. 2010; 95:1651–1659.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download