Abstract
Renovascular hypertension is caused by narrowing of the arteries supplying the kidneys. There are several methods to treat renal artery stenosis, such as medications, percutaneous transluminal renal angioplasty, and atherosclerosis. A boy presented to our hospital with severe hypertension. Computed tomography angiogram revealed severe narrowing of the left renal artery and hypoplastic left kidney. Total renal artery embolizaton was performed to make a complete occlusion of the left renal artery. Follow-up renin and aldosterone levels were gradually decreased. The main advantage of renal artery embolization is that it is minimally invasive compared with extensive surgical procedures. Therefore, renal artery embolization should be considered as an alternative to surgical nephrectomy in pediatric patients with renovascular hypertension.
Renal artery stenosis (RAS) is the most important cause of renovascular hypertension and treatable secondary hypertension in childhood.1 However, renovascular hypertension results not only from reduced renal perfusion due to RAS but also from impaired renal parenchyma and function due to renal hypoplasia, activating Renin-Angiotensin-Aldosterone system.2
Recently, various methods such as stent placement and balloon angioplasty have been tried to correct symptomatic RAS.3 This report describes a case of a renal artery stenosis with renal hypoplasia treated by transcatheter coil embolization.
A 9-year-old boy was referred to our hospital due to uncontrollable arterial hypertension. He was the second baby from twin gestation in a female who underwent in vitro fertiliazation pre-embryo transfer and was prematurely born at 30 weeks and 1 day gestational age with birth weight of 1580 gm. On the 10th day of admission in the neonatal intensive care unit, he presented with elevated blood pressure which was attributed to umbilical cord catheterization. Elimination of the umbilical catheterization and three-day medication with captopril led the high blood pressure to subside. Renal ultrasonography including Doppler interrogation revealed that the size of both kidneys was different from each other (right kidney 4.4×1.7 cm, left kidney 3.6×1.5 cm) and the renal blood flow was decreased in the left kidney. He was discharged with medication of hydralazine and captopril. Although he was recommended to regularly attend outpatient clinic, he was follow-up lost since then.
Seven years later, he visited a local clinic due to repetitive epistaxis and was referred to the department of pediatric nephrology for the evaluation of uncontrollable hypertension, measuring between 220/100 and 140/90 mm Hg. 274On admission, his body weight was 25 kg (10th percentile) with a height of 132.9 cm (25-50th percentile), a pulse rate of 96 beats per minute, and a blood pressure of 120/75-122/85 mm Hg in all four limbs at rest. There were no abdominal or carotid murmur and no palpable masses in his abdomen. The clinical and neurological examination was normal. The echocardiography showed left ventricular hypertrophy with a left ventricular mass of 116 g/m2 (range, 70-80). The left ventricular function was normal with ejection fraction of 73%, and the aortic arch showed no abnormality. There was no evidence of aortic coarctation or no obvious diastolic dysfunction.
Blood chemistry values including creatinine, sodium, potassium, and chloride were unremarkable. Additional endocrine tests showed normal values for thyroid hormones. The renin level was >20 ng/mL/hr (range, erect 1.9-6.0), aldosterone level of 44.84 pg/mL (range, 12-340 for age), cortisol level of 10.2 ug/dL (range, 3.1-12.7), vanillylmandellic acid level of 2.68 mg/day (range, 0.0-8.0), and homovanillic acid level of 5 mg/day (range, 0.0-8.0). Urinalysis was within normal limit without proteinuria, hematuria, and renal tubular dysfunction.
In abdomenal computed tomography (CT), the left hypoplastic kidney was observed, with narrow and tortuous left renal artery, indicating congenital left renal hypoplasia or secondary contracted left kidney (Fig. 1). On the captopril-primed diethylene triamine pentaacetic acid scan, the early uptake, perfusion, parenchymal transition time, and excretion were all delayed in the left kidney, as well as the decreased left kidney in size (Fig. 2). In contrast, all the values in the right kidney were normal.
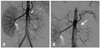
Taking a renal arteriogram, coil embolization was performed in the left two renal arteries. At the end of the procedure, another arteriogram confirmed the complete suppression of arterial flow to the left kidney (Fig. 3). While performing renal arterial embolization, blood was sampled in both renal veins to measure renin levels again. The renin levels in the right and left renal veins were 6.67 ng/mL/hr and above 20 ng/mL/hr, respectively. After the procedure, there was no uptake in the left kidney on dimercaptosuccinic acid (DMSA) scan, showing complete occlusion of the left renal artery (Fig. 2). The embolization caused mild pain in the left flank and slight fever in the patient, which were resolved in one week. Before discharge, we remeasured renin level, and it was 6.76 ng/mL/hr. His blood pressure was maintained between 125/75 and 135/85 mm Hg, and he was discharged with oral medication (amlodipine 5 mg q.d. and angiotensin receptor blocker 50 mg q.d.) for one month.
One month later, his blood pressure returned to normal, 95/66-125/75 mm Hg. His renin and aldosterone levels were 7.71 ng/mL/hr and 23.94 pg/mL, respectively. Follow-up abdominal CT showed enhancement in the cortex of the left contracted kidney, suggesting collateral blood that supplies to the left kidney. However, his blood pressure was maintained in the normal range without any antihypertensive medications.
Renovascular hypertension is mostly due to conditions such as fibromuscular dysplasia or vasculitis; for example, Takayasu arteritis and polyarteritis nodosa.4-6 However, the pathogenesis of renovascular hypertension is complicated and is mainly due to the overactivation of Renin-Angiotensin-Aldosterone system, consequently causing ischemic nephropathy and eventually interstitial fibrosis.1
There are several methods to treat renal artery stenosis, including surgery, percutaneous transluminal renal angioplasty, balloon angioplasty, and renal artery stenting. Among these various methods, renal artery embolization was selected for our patient because his renal artery stenosis was entirely due to renal hypoplasia and this procedure was minimally invasive.
Renal embolization was initially described in adult patients, mainly for the treatment of neoplasms.7 Renal embolization is indicated in the patients with end-stage kidneys and uncontrollable hypertension or massive proteinuria.7 Other indications are the ablation of native kidneys in transplant recipients and an irreversibly rejected allograft. Surgical nephrectomy is not preferred in many children, because of the patient's poor general condition or invasiveness.4,7 However, dilatation and stenting are not a adequate method because of the very small vessel size (1-2 mm diameter) and potentially high restenosis rate due to recoil of the stenosis.8
In conclusion, renal artery embolization can be an alternative to surgical nephrectomy in pediatric patients with RAS and renal hypoplasia, which is minimally invasive, compared with other extensive surgical procedures and can also efficiently control high blood pressure.
Figures and Tables
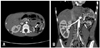 | Fig. 1Computed tomography, abdomen. Horizontal view (A) and coronal view (B) showing left renal arteries stenosis and hypoplastic left kidney (white arrows). |
 | Fig. 2Captopril-primed diethylene triamine pentaacetic acid scan (A) shows that the early uptake, perfusion, parenchymal transition time, and excretion were all delayed in the left kidney, as well as the decreased left kidney in size before embolization. 99 m Technetium-2, 3 dimercaptosuccinic acid scan (B) reveals no uptake in the left kidney, performed seven days after embolization. |
References
1. Yerram P, Karuparthi PR, Chaudhary K. Pathogenesis and management of renovascular hypertension and ischemic nephropathy. Minerva Urol Nefrol. 2012; 64:63–72.
2. Vogt B, Maillard M, Burnier M. Hypertension therapy in patients with renal artery stenosis. Ther Umsch. 2012; 69:279–281.
3. Colyer WR, Eltahawy E, Cooper CJ. Renal artery stenosis: optimizing diagnosis and treatment. Prog Cardiovasc Dis. 2011; 54:29–35.

4. Capozza N, Collura G, Falappa P, Caione P. Renal embolization as an alternative to surgical nephrectomy in children. Transplant Proc. 2007; 39:1782–1784.

5. Reuter SR, Pomeroy PR, Chuang VP, Cho KJ. Embolic control of hypertension caused by segmental renal artery stenosis. AJR Am J Roentgenol. 1976; 127:389–392.

6. Ishijima H, Ishizaka H, Sakurai M, Ito K, Endo K. Partial renal embolization for pediatric renovascular hypertension secondary to fibromuscular dysplasia. Cardiovasc Intervent Radiol. 1997; 20:383–386.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download