Abstract
Purpose
To compare the epithelial wound healing response of two preservative-free fluoroquinolones, moxifloxacin and levofloxacin, in patients who underwent cataract surgery.
Materials and Methods
In this prospective, evaluator-masked, randomized clinical trial, 59 eyes of 50 patients who underwent cataract surgery were enrolled. Patients were randomized to receive moxifloxacin 0.5% (n=32 eyes) or levofloxacin 0.5% (n=27 eyes). All patients instilled moxifloxacin or levofloxain four times daily for 1 week prior to surgery and 2 weeks after surgery. The epithelial wound healing status in the corneal incision site was scanned with a raster scan mode of fourier-domain optical coherence tomography (FD-OCT). The number of eyes showing epithelial defect images and average number of corneal epithelial defect cuts per eye were compared between groups. All patients were evaluated on postoperative days 1, 2, 3, and 10.
Results
On postoperative days 1, 2, and 3, the number of eyes showing epithelial defects in FD-OCT was not statistically different (all p>0.05). The average number of corneal epithelial defect cuts was also not statistically different between the two groups (all p>0.05). No eyes showed epithelial defects on postoperative day 10 in either group.
The selection of appropriate antibiotic eye drops is critical for preventing endophthalmitis, one of the most detrimental complications after cataract surgery. Fluoroquinolones have long been used for the prevention of postoperative infections, but resistance to third-generations fluoroquinolones led to the introduction and development of fourth-generation fluoroquinolones (moxifloxacin or gatifloxacin). Moxifloxacin and gatifloxacin have demonstrated 2- to 3-fold penetration into the anterior chamber, improved activity against atypical mycobacteria, and greater antibacterial effects against gram-positive bacteria compared to second-generation (ciprofloxacin and ofloxacin) or third-generation (levofloxacin) fluoroquinolones.1,2 Adverse epithelial cytotoxic effects, however, should also be considered when choosing antibiotics because rapid re-epithelialization helps to prevent microbial invasion and other possible complications such as epithelial ingrowth.3,4
Moxifloxacin and levofloxacin are commonly used antibiotic eye drops after cataract surgery or refractive procedures. Although there have been several reports comparing effects on epithelial wound healing of various fluoroquinolones as well as their potential cytotoxicity,5-8 the results were not consistent. These studies also compared fluoroquinolones that contain the preservative, benzalkonium chloride (BAK), with fluoroquinolones without BAK. BAK itself has been reported beneficial in helping to prevent bacterial growth at a rate faster than preservative-free fluoroquinolones, especially against gram-positive bacteria.9 However, BAK is known to cause corneal and/or conjunctival epithelial toxicity.10,11 Moreover, to our knowledge, there is no study comparing these two types of topical fluoroquinolones in human eyes after cataract surgery.
To compare the intrinsic effects of third- and fourth-generation fluoroquinolones without the influence of BAK on the re-epithelialization of corneal incision sites after cataract surgery, we compared the effects of two commercially available preservative-free fluoroquinolones, moxifloxacin (Vigamox®, Alcon Laboratories, Fort Worth, TX, USA) and levofloxacin (Cravit®, Santen, Osaka, Japan).
This study was a prospective, single-masked, randomized, comparative evaluation of patients undergoing phacoemulsification. All participants signed an informed consent after a detailed explanation of the study. This study was prospectively approved by the Institutional Review Board of Severance Hospital and followed the tenets of the Declaration of Helsinki.
The investigator assigned the operated eye of each patient with an identification number that corresponded to a treatment regimen based on a randomization scheme created at baseline. The patients then received the appropriate treatment regimen for their identification number based on this randomization scheme. This allowed the investigator to be blinded as to which treatment regimens were allocated to patients. To minimize inter-surgeon variability, a single surgeon (E.K.K.) performed all surgical procedures. A single investigator (W.S.C.), under the same conditions, evaluated the postsurgical wound.
The inclusion criteria for this study were patients >18 years age who were scheduled for removal of a cataract and implantation of a posterior chamber intraocular lens (IOL), intraocular pressure ≤20 mm Hg prior to surgery, and had otherwise normal and healthy eyes aside from cataracts as determined by ophthalmic examination. Patients with any of the following conditions were not eligible to participate in this clinical trial: fluorescein staining of the cornea before surgery, history of any ocular diseases including ocular inflammatory diseases, ocular herpes infection, iritis, uveitis, Sjögren's syndrome, corneal dystrophy, uncontrolled diabetes and/or diabetic retinopathy, any history of systemic disease such as autoimmune diseases, history of treatment for an ocular infection within 30 days prior to study entry, use of topical or systemic steroids within 7 days prior to study entry, use of topical anti-inflammatory drugs within 7 days prior to study entry, known or suspected allergy or hypersensitivity to levofloxacin or any related medicines, such as cinoxacin, ciprofloxacin, norfloxacin, ofloxacin, or nalidixic acid, preservatives, dyes, or any component of the medications involved in the study, pregnancy, nursing/lactation, or inadequate birth control methods. Oral contraceptive use was allowed. Patients with any cataract wounds requiring sutures or corneal burn secondary to the phacoemulsification handpiece were excluded in this study.
Patients were instructed to use the assigned antibiotics, moxifloxacin or levofloxacin, four times a day for 1 week prior to surgery. Standard phacoemulsification with a 2.2 mm clear corneal 3-plane incision was performed. Each corneal incision was made with a disposable phacoblade (Slu-22AGF®, Kai medical, Gifu, Japan). The length of the corneal incision was set at 2 mm or greater for self-sealing. A 2.2 mm microcoaxial phaco-handpiece (No. 8065750853, Alcon, Fort Worth, TX, USA) was used for phacoemulsification. After irrigation and aspiration of the cortex, a posterior chamber intraocular lens (Acrysof SN60WF, Alcon, Fort Worth, TX, USA) was inserted into the capsular bag. At the end of surgery, the corneal wound was sealed with stromal hydration. If any wound leak was observed, the corneal wound was sutured with 10-0 nylon sutures, and the patient was excluded from the study. An eye patch was then placed over the for 6 hours and then removed. Patients were asked to instill the assigned antibiotic four times per day. Prednisolone acetate 1% (Pred-Forte®, Allergan, Inc., Irvine, CA, USA) was also instilled four times per day concurrently. After 2 weeks, eye drop use was discontinued. Patients were evaluated on postoperative days 1, 2, 3, and 10.
Epithelial defect size can vary in accordance with elapsed time after staining and strength of illumination (Fig. 1A). To obtain more objective information with regards to both the length and depth of epithelial defect, the corneal incision site was scanned with the raster scan mode of FD-OCT, which provides 17 cross-sectional images in a size adjustable square that measured 3×3 mm2, resulting in 17 focal points each measured at 0.187 mm intervals in that square (Fig. 1B).
An epithelial defect cut was defined when an interruption or lack of epithelial growth (a black hollow space in the incision site, white arrows in Fig. 2A-1 and A-2) was found in the FD-OCT image above the extension line, an imaginary extension line between the cut edges of Bowman layer (red dotted line of Fig. 2A and B). Images showing a continuous epithelial growth (a dark gray space connected with the epithelium which showing similar dark gray color, black arrows in Fig. 2B-1 and B-2) above this line without interruption were interpreted as a non-defect cut (Fig. 2B).
In eyes showing epithelial defects, the degree of wound healing was determined based on the number of defect cuts on the FD-OCT image (Fig. 1C). The proportion of eyes that showed epithelial defects and the average number of defect cuts (the total number of defect cuts found in each group/the number of patients with defect cuts in each group) were compared between the two groups.
All data was analyzed by a statistician not involved in the study design or collection of data. Statistical analyses were performed using PASW for Windows (version 18.0, SPSS Inc., Chicago, IL, USA). Average values of parameters were compared using either the chi-square test or Fisher's exact test. A p value less than 0.05 was considered statistically significant.
Eighty-four eyes of 72 patients were initially included. Nine patients receiving cataract surgery on both eyes had a period of at least two weeks between each cataract surgery, with each eye being treated as a new case. Among the initial 84 eyes, 25 eyes were excluded for the following reasons: cancellation of surgery due to personal reasons (15 eyes), suture placement after surgery due to wound leakage (nine eyes), and occurrence of posterior capsule rupture (one eye). Finally, 59 eyes of 51 patients were enrolled upon completion of the study; 32 eyes received moxifloxacin and 27 eyes received levofloxacin. The mean age of the 51 patients was 65.1±8.0 years (moxifloxacin group; 65.4±7.4 years, levofloxacin group; 64.6±8.7 years, p>0.05). There were no postoperative complications in these patients during the 10 days of follow-up after successful phacoemulsification and IOL implantation.
On the first day after the operation, 15 of 32 eyes (46.9%) of the moxifloxacin group and 17 of 27 eyes (63.0%) of the levofloxacin group showed at least one corneal epithelial defect cut, but the difference was not statistically significant (p=0.217, chi-square test) (Table 1). On postoperative day 2, five eyes (15.6%) in the moxifloxacin group and one eye (3.7%) in the levofloxacin group showed corneal epithelial defect cuts, but the difference was not statistically significant (p=0.205, Fisher's exact test). On postoperative day 3, one eye (3.1%) in the moxifloxacin group showed corneal epithelial defect cuts and there was no eye showing an epithelial defect cut in the levofloxacin group. There was no statistically significant differences between the two groups (p=0.542, Fisher's exact test). On postoperative day 10, no eye had an epithelial defect cut in either group.
Because individual variance of epithelial defect size existed, we evaluated the mean number of epithelial defect cuts per eye (the total number of epithelial defect cuts/the number of eyes with epithelial defect) and compared the values between the groups.
On postoperative day 1, the mean number of defect cuts per eye was 3.93±2.34 (range: 1-9 per eye) in the moxifloxacin group and 2.82±1.67 (range: 1-7 per eye) in the levofloxacin group. On postoperative day 2, the mean number of defect cuts per eye was 1.60±1.34 (range: 1-5 per eye) in the moxifloxacin group and 1 (1 cut/1 eye) in the levofloxacin group. On postoperative day 3, the mean number of defect cuts per eye was two (2 cuts/1 eye) in the moxifloxacin group and none (0 cut/0 eye) in the levofloxacin group. On postoperative day 10, there was no defect cut in either group. There were no statistically significant differences through the follow-up examinations (all p>0.05).
In this study, there were no significant differences in the number of eyes showing epithelial defects between the moxifloxacin and levofloxacin groups on days 1, 2, 3, and 10 after cataract surgery. There was also no statistical difference in the average number of defect cuts per eye between the two groups on follow-up examinations.
Numerous experimental and clinical studies have compared the cytotoxicity of different fluoroquinolones, including levofloxacin and moxifloxacin, but the results have been inconsistent. In contrast to our results, Kim, et al.12 reported that levofloxacin (Cravit®) was less cytotoxic than moxifloxacin (Vigamox®) when these eyedrops were exposed to cultured human corneal epithelial cells over 2 hours. Tsai, et al.10 incubated human corneal epithelial cells with different fluoroquinolones (norfloxacin, ciprofloxacin, ofloxacin, levofloxacin, moxifloxacin and gatifloxacin) using both commercial ophthalmic formulations and raw materials (standard powders) of each antibiotic, and reported that levofloxacin and ofloxacin showed the least cytotoxicity. In contrast, Sosa, et al.11 reported that among moxifloxacin, gatifloxacin, ofloxacin, and levofloxacin, moxifloxacin showed the lowest degree of cytotoxicity in immortalized conjunctival and human corneal epithelial cells. In a clinical study, Kovoor, et al.13 compared in vivo confocal microscopy images after instillation of either moxifloxacin, ofloxacin, levofloxacin, gatifloxacin, or ciprofloxacin on healthy rabbit corneas, and showed that moxifloxacin caused the least damage to the corneal epithelium. Nevertheless, in the last two studies mentioned, moxifloxacin was the only solution that did not contain BAK. Because the effect of BAK was not excluded in those studies, the authors presumed the cytotoxicity of fluoroquinolones was mainly a result of the preservatives and not of the fluoroquinolone itself.
To exclude these interfering effects of BAK, we have chosen commercially available and preservative-free moxifloxacin and levofloxacin, and our results showed there were no statistical differences in epithelial wound healing in clear corneal incision. This is consistent with an in vivo study that used the same preservative-free fluoroquinolones as we studied, in which Watanabe, et al.14 treated healthy human volunteers with either moxifloxacin or levofloxacin three times per day for 1 week and reported that there were no differences in tear break-up time and morphological appearance of the corneal epithelium, stroma, and endothelium.
In this study, we used a clear corneal incision model to compare the effect of two different topical fluoroquinolones on wounded human corneas. As these patients were already scheduled for cataract surgery, the corneal incisions in this study posed no ethical problems and it was an appropriate model that could induce the standardized form of epithelial damage. Through this model, epithelial defects of the same length were made, and the rate of re-epithelialization could be compared.
Accurate epithelial defect measurements could not be ascertained when using fluorescein dye staining because sometimes distinguishing between true epithelial defect and wound irregularity after epithelial healing is difficult. In addition, quantitative measurements of the degree of wound healing, as shown in Fig. 1A, can also be difficult. In this study, we used anterior segment FD-OCT to measure epithelial defects. Using FD-OCT, we could quantitatively measure the epithelial defect from focal points measured at 0.187 mm intervals. Thus, the length of epithelial defect could be measured more accurately.
The current study is the first to compare corneal epithelial wound healing in human eyes after cataract surgery with topical use of moxifloxacin and levofloxacin. Our study showed that there was no significant difference between these two antibiotics in terms of their effect on re-epithelialization after cataract surgery.
Figures and Tables
Fig. 1
(A) A slit lamp photograph of the corneal incision after fluorescence dye staining. The margin of corneal defect is obscure (a or b) and can be interpreted differently according to varying strengths of illumination. (B) A capture window of raster scan mode in fourier-domain optical coherence tomography (FD-OCT). By selecting a 3×3 mm2 sized capture window, 17 cuts of cross-sectional images (white and red arrows) could be acquired at the same gap. (C) On cross-sectional images of red arrows in Fig. 1B, the defect cuts (C-1 to 4) can be counted. The actual margin of corneal defect detected based on the FD-OCT images was determined to be "a" not "b" in Fig. 1A.
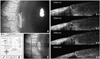
Fig. 2
(A) Representative images showing the defect and non-defect image cuts in images of the fourier-domain optical coherence tomography. (A and B) An imaginary line (red dotted line) was drawn connecting Bowman layer on both ends of the sectional plane. (A-1 and A-2) Cases showing a lack of epithelium on the imaginary line and a discontinuation of this line were interpreted as a defect cut. The black hollow spaces without epithelial growth were noted (white arrows). The layer of powerfully bright light is considered to be a reflection of the tear fluid (white hollow arrowheads). (B-1 and B-2) Cases showing epithelial growth both above this line and without interruptions were interpreted as a non-defect image cut. The spaces in the incision site were occupied with epithelial growth which showing similar dark gray color with the epithelial layer (black arrows).
ACKNOWLEDGEMENTS
This research was supported by the Converging Research Center Program funded by the Ministry of Education, Science and Technology (2012K001354) and by the educational grant from Alcon Laboratories, Inc. (Fort Worth, TX, USA).
All patients gave informed consent for participation in research. This study is registered at http://www.clinicaltrials.gov (NCT00840580).
Terry Kim is a surgical consultant for Alcon, Inc.
References
1. Hooper DC, Wolfson JS. Mode of action of the quinolone antimicrobial agents. Rev Infect Dis. 1988; 10:Suppl 1. S14–S21.

2. Mather R, Karenchak LM, Romanowski EG, Kowalski RP. Fourth generation fluoroquinolones: new weapons in the arsenal of ophthalmic antibiotics. Am J Ophthalmol. 2002; 133:463–466.

3. Vargas LG, Vroman DT, Solomon KD, Holzer MP, Escobar-Gomez M, Schmidbauer JM, et al. Epithelial downgrowth after clear cornea phacoemulsification: report of two cases and review of the literature. Ophthalmology. 2002; 109:2331–2335.

4. Lee BL, Gaton DD, Weinreb RN. Epithelial downgrowth following phacoemulsification through a clear cornea. Arch Ophthalmol. 1999; 117:283.

5. Pollock GA, McKelvie PA, McCarty DJ, White JF, Mallari PL, Taylor HR. In vivo effects of fluoroquinolones on rabbit corneas. Clin Experiment Ophthalmol. 2003; 31:517–521.

6. Patel GM, Chuang AZ, Kiang E, Ramesh N, Mitra S, Yee RW. Epithelial healing rates with topical ciprofloxacin, ofloxacin, and ofloxacin with artificial tears after photorefractive keratectomy. J Cataract Refract Surg. 2000; 26:690–694.

7. Moreira LB, Lee RF, de Oliveira C, LaBree L, McDonnell PJ. Effect of topical fluoroquinolones on corneal re-epithelialization after excimer laser keratectomy. J Cataract Refract Surg. 1997; 23:845–848.

8. Moshirfar M, Chew J, Werner L, Meyer JJ, Hunter B, Stevens S, et al. Comparison of the effects of fourth-generation fluoroquinolones on corneal re-epithelialization in rabbit eyes. Graefes Arch Clin Exp Ophthalmol. 2008; 246:1455–1461.

9. Kowalski RP, Kowalski BR, Romanowski EG, Mah FS, Thompson PP, Gordon YJ. The in vitro impact of moxifloxacin and gatifloxacin concentration (0.5% vs 0.3%) and the addition of benzalkonium chloride on antibacterial efficacy. Am J Ophthalmol. 2006; 142:730–735.

10. Tsai TH, Chen WL, Hu FR. Comparison of fluoroquinolones: cytotoxicity on human corneal epithelial cells. Eye (Lond). 2010; 24:909–917.

11. Sosa AB, Epstein SP, Asbell PA. Evaluation of toxicity of commercial ophthalmic fluoroquinolone antibiotics as assessed on immortalized corneal and conjunctival epithelial cells. Cornea. 2008; 27:930–934.

12. Kim SY, Lim JA, Choi JS, Choi EC, Joo CK. Comparison of antibiotic effect and corneal epithelial toxicity of levofloxacin and moxifloxacin in vitro. Cornea. 2007; 26:720–725.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download