Abstract
Purpose
The aim of this study was to evaluate the effect of surgical repair of pelvic organ prolapse on female sexual function and to assess correlations between the two using two current standardized questionnaires.
Materials and Methods
From October 2009 to September 2010, 143 patients with posterior compartment or combined vaginal prolapse were included. We assessed surgical outcomes according to anatomical change in the vagina and results of the Female Sexual Function Index (FSFI) and Pelvic Organ Prolapse/Urinary Incontinence Sexual Function questionnaire (PISQ-12) both pre- and postoperatively.
Results
Among the 143 preoperative patients, 99 and 84 patients responded to the PISQ-12 and FSFI, respectively. The mean PISQ-12 score increased after surgery (p<0.001). Specifically, postoperative scores for questions 8 and 12 were higher than their respective preoperative scores (p<0.001). Postoperatively, mean FSFI score changed only slightly (p=0.76), and only the score for the satisfaction domain was improved (p=0.023). In regards to vaginal anatomy, vaginal length was significantly greater postoperatively (6.99±0.18 vs. 7.56±1.08, p<0.001), and postoperative vaginal caliber was narrowed to a two-finger width.
Sexual well-being is an important aspect of women's health, and dysfunction thereof has been shown to lead to a decreased quality of life. Sexual dysfunction among women has been reported to be highly prevalent in up to 50% of women,1 and disorders of desire, arousal, lubrication, and orgasm as well as dyspareunia are typical complaints reported by women.2 Furthermore, several factors, including relationship conflicts, socioeconomic status, sexual compatibility, and physical and psychiatric disorders can affect sexual function.3-5 Pelvic floor weakness and organ prolapse are common physical conditions that negatively affect sexual function and satisfaction.4,5 Pelvic organ prolapse occurs in greater than 50% of parous women over 50 years of age and poses a lifetime risk of 30-50%.6 Patients with clinically significant pelvic organ prolapse usually complain of a vaginal bulging sensation accompanied by symptoms of bladder, bowel, or sexual dysfunction.7
To treat pelvic organ prolapse, both surgical and nonsurgical modalities are available. Conservative treatment options, including observation, pelvic floor exercises, and vaginal pessaries, usually in combination with local estrogen,2 are recommended before turning to surgical treatment, even though there are 20-40% rate of re-operation for prolapse of the anterior compartment, 5-20% for the posterior compartment, and up to 30% for the apical compartment.8 Although most cases are managed surgically,9 earlier studies have shown contradictory results: a few authors reported that surgical treatment of pelvic floor weakness and pelvic organ prolapse improves sexual function postoperatively,10 while others reported worsening of sexual function.11,12
The aim of this study was to compare the effect of surgical repair of pelvic organ prolapse on female sexual function with the results of two different standardized questionnaires administered to Korean women.
Between October 2009 and September 2010, we asked all patients scheduled to undergo surgery for International Continence Society Pelvic Organ Prolapse Quantification (ICS POP-Q) stage II-IV pelvic organ prolapse13 at Yonsei University Severance Hospital to participate in this study. Our study was approved by the hospital's Institutional Review Board, and all patients provided informed consent to participate in the study. Preoperative and postoperative pelvic assessments were performed by the same surgeon according to the ICS POP-Q staging system. In this study, patients with posterior compartment or combined vaginal prolapse who required posterior colporrhaphy and perineorrhaphy were enrolled. Patients with prolapse of the anterior or superior compartments only and those who did not respond to the questionnaires were excluded. Preoperative and postoperative subjective urinary, coital, and bowel symptoms were assessed by interview. All enrolled patients underwent a physical examination, including gynecologic examination and preoperative urodynamic measurements to evaluate occult stress urinary incontinence (SUI). Patients also provided information on their general medical and sexual history.
In this study, the Female Sexual Function Index (FSFI) and Pelvic Organ Prolapse/Urinary Incontinence Sexual Function questionnaire (PISQ-12) were administered to evaluate sexual function. Patients were required to complete the Korean version of questionnaires translated from English prior to and after undergoing surgery. Postoperative assessment was carried out 36 weeks after the operation. The questionnaire was self-administered; beforehand, the interviewer thoroughly explained each questionnaire and all questions. Total 4 cases (4.7% vs. 4%) couldn't report by themselves because of their illiteracy. In cases where the interviewee was unable to read or write, the interviewer just read the questions aloud and recorded the patient's reply to limit bias. All questionnaires were surveyed by the same interviewer. Questionnaires that contained responses to all items were evaluated, whereas questionnaires with blanks were excluded from the evaluation.
Standardized questionnaires including FSFI and PISQ-12 were utilized to assess sexual function in women with pelvic organ prolapse. The FSFI is a 19-item survey that assesses six domains of female sexual dysfunction (FSD). Scores range of the FSFI from 2 to 36.0; a total score of 26.55 or less is suggestive of FSD and individual domain scores of less than 3.6 are considered dysfunction.14 The FSFI assesses sexual function over the previous six months and has been confirmed for the Diagnostic and Statistical Manual of Mental Disorders IV diagnoses of FSD including hypoactive sexual desire disorder, female sexual arousal disorder, and female sexual orgasmic disorder.15
In contrast, the original PISQ comprises 31 questions including three domains to be reflective of sexual function over the previous four weeks: behavioral-emotive, physical, and partner-related.16 The first domain, the behavioral-emotive session, examines the frequency of sexual activity, the desired frequency, orgasm rates, and satisfaction with one's sexual relationship.16,17 In second, episodes of pain, incontinence, sensation of prolapse, and fear of fecal and/or urinary incontinence during sexual activity are included the physical domain.16,17 The partner-related domain lastly measures any difficulty with erectile dysfunction, premature ejaculation, vaginal attenuation, vaginal tightness or the patient's perception of a partner's avoidance of intercourse.16,17 Among of 31 questions, PISQ-12 were made of the most validated 12 questions only as short form.17
Our study examined changes in sexual function based on patient responses to these questionnaires following surgery for pelvic organ prolapse. Furthermore, after a systemic search on MEDLINE (search terms: "sexual function including the FSFI and the PISQ-12 for surgically treated pelvic organ prolapse/urinary incontinence", in all languages, from 1970 to 2010), several studies reporting on sexual function after prolapse repair were found and included in our analysis if they employed the FSFI or PISQ-12.
Statistical comparisons were made using the Wilcoxon signed-rank test for vaginal anatomy, each domain of sexual function, and changes in FSFI and PISQ-12 scores. SPSS software version 19.0 (SPSS Inc., Chicago, IL, USA) was used to analyze the data. p<0.05 was considered to be statistically significant. A correlation analysis and chi-square test were used to evaluate categorical data. A covariance mixed model was used to control for confounding variables in the analysis of PISQ-12 score, FSFI score and individual factors. These were evaluated with SAS software (version 9.2, Cary, NC, USA).
In total, 143 women with a mean age of 65.13±9.99 years were included in the study. A total of 84 patients completed the FSFI and 99 patients completed the PISQ-12. The baseline demographic and clinical parameters of the patients are shown in Table 1. In this study, the primary outcome was change in vaginal anatomy including total vaginal length (TVL) and genital hiatus (GH). To compare changes in vaginal anatomy, TVL and GH measurements were taken preoperatively and six months after surgery at our outpatient clinic by the same surgeon. GH was defined as the largest diameter at the introitus. TVL was defined as the distance from the tip of the ringed forceps at the vaginal apex to the posterior vaginal introitus. Postoperatively, total vaginal length increased from 6.99±0.19 cm to 7.56±1.08 cm in TVL (p<0.001) and genital hiatus narrowed from 5.01±0.98 cm to 3.00 cm (p<0.001).
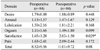
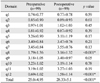
The secondary outcome was the difference between preoperative and postoperative FSFI and PISQ-12 scores. The total FSFI score was 8.53±9.5 before surgery and 11.02±9.7 at 6 months after surgery (p=0.08), and only the satisfaction domain score significantly increased (p=0.029). In our study, 82 of 84 cases (97.6%) were classified as having FSD with a score of 26.55 on FSFI. The results of the FSFI indicated that satisfaction was improved (Table 2). The mean preoperative PISQ score changed from 25.0±4.95 to 28.33±5.1 postoperatively (p<0.001); more specifically, postoperative scores for questions 8 (q8) (Do you avoid sexual intercourse because of bulging in the vagina?) and 12 (q12) (Compared to orgasms you have had in the past, how intense are the orgasms you have had in the past six months?) were higher than their respective preoperative scores (p<0.001). In other words, patients reported that they were less likely to avoid sexual intercourse due to vaginal prolapse (q8) (1.79 vs. 3.76 postoperatively, p<0.01) after surgery. The orgasm score (q12) on the PISQ-12 was also increased after surgery (0.69 vs. 1.58, p<0.01); however, results indicated that orgasm intensity was the same after surgery, corresponding to a score of approximately 2 (Table 3). No correlation among the scores of the PISQ-12, FSFI and pelvic organ prolapse stage (Table 2 and 3) was observed.
Statistical analysis was performed to identify cofactors affecting these results. Correlations between each domain of the FSFI and PISQ-12 as well as patient variables were assessed using univariate analysis and a covariance patterned model. On the FSFI, the desire and arousal domains were related to parity, menopause, weight and body mass index (BMI). Lubrication and orgasm were related to menopause, weight and BMI. Satisfaction was associated with menopause and weight. Parity, menopause, previous hysterectomy and concomitant SUI affected the pain domain. Overall scores were related to parity, menopause, weight and BMI (Table 4). On the PISQ-12, overall score was influenced by menopause, smoking and hormonal replacement therapy (HRT). Q1 to q12 were affected by cofactors including parity, menopause, metabolic syndrome, HRT, etc. Concomitant SUI affected a variety of sexual activities (q4) and leakage of urine during sexual activity (q6), but it did not affect the change in the total score (Table 3 and 5). In the adjustment model, menopause was the only cofactor shown to affect postoperative sexual function (Table 6 and 7).
FSD is a common problem among the general population, and especially in pelvic organ prolapse patients. In the literature, a few studies are found to have evaluated the impact of pelvic floor surgery on FSD,18 however, significant controversy remains as the actual benefits thereof.19 While some authors reported normal or even improved sexual activity following pelvic organ prolapse surgery, other authors reported no difference or worsening of sexual function.2,6,11,12,20 In fact, recent studies have clearly shown that there are no differences in sexual function between patients with and without pelvic floor disease, including urinary incontinence and/or pelvic organ prolapse, with over 81% of sexually active women with prolapse defining their sexual relations as "very satisfactory".2,6,20
For patients affected by pelvic organ prolapse, the goals of pelvic reconstructive surgery are to maintain the natural vaginal axis and to restore the original vaginal structure including vaginal length, vaginal orifice and perineum. In our study, vaginal anatomy, consisting of vaginal length, perineal body and orifice width, was about the same after pelvic reconstructive surgery. These findings made our points clear on the association between cofactors and female sexual function postoperatively.
In the literature, earlier reports describe worsening of pain.11,20 Narrowing of the vaginal orifice can occur with colporrhaphy and can lead to de novo dyspareunia even if there is an improvement in sexual function.20 However, the pain domain in this study was unchanged postoperatively. Hoda, et al.21 reported that worsening of pain during intercourse throughout the initial postoperative period lessened after three months as healing progressed; moreover, dyspareunia peaked three months after surgery and then decreased. In this study, we expected that patients responding at six months after surgery would complain of dyspareunia. Due to the time period during six months covered by the survey and the patients' ability to recall, FSFI responses reflected a reduction in dyspareunia and PISQ-12 responses reflected only relief of pain. Concomitant anti-incontinence surgery can deteriorate some sexual domains. Transobturator tape may affect an orgasmic domain because it is able to pass the obturator foramen through the dorsal nerve of the clitoris.22,23 For this procedure, vaginal dissection may damage the distal perineal and cavernous nerves, negatively affecting the innervation of the clitoris and altering its sensitivity.24 Other studies reported that anti-incontinence surgeries could damage vascular structures, alter normal vaginal anatomy, induce scarring, and reduce the elasticity of vaginal wall, causing dyspareunia.25,26 In our study, concomitant anti-incontinence procedures were revealed to be a cofactor of q4 (a variety of sexual activities) and q6 (leakage of urine during sexual activity) despite no statistical improvement to each item. Interestingly, sling procedures could affect dyspareunia as cofactor without change of total score on the FSFI. In summary, sexual function scores as measured by FSFI and PISQ-12 were unchanged following pelvic reconstructive surgery with or without anti-incontinence surgery, despite improvements in the stage of prolapse incontinence symptoms. Recent studies found that sexual function was unchanged following reconstructive surgery with or without a surgical procedure for concomitant SUI.27,28 For the sexual function, alternative therapy including botulinum toxin injection or periurethral injection with bulking agent might be considered a treatment of concomitant urinary incontinence. However, efficacy of these alternative treatments is controversial.29-32 Therefore, it is not yet considered to be the first line therapy for anti-incontinence surgery.
Increasing age is known to be related to a reduction in sexual activity and decreased availability of partners.6 As older age is generally associated with FSD, differences in mean age in prior studies have made comparisons between studies imprecise. The population in the present study was significantly older than the population studied by previous authors.13-17 Preoperative FSD was diagnosed in 82 out of 84 (97.6%) patients based on a score of 26.55 or below on FSFI. The proportion with FSD in the present study was much higher than that in other studies.13-17 In our study, the number of patients with FSD postoperatively was same as that preoperatively. Similar to Barber, et al.,4 pelvic reconstruction did not change the number of patients who were sexually active. We also found menopause to be a cofactor of FSD, but this has not yet been shown to be related to decrease of sexual function in the literature.6,13-17 This could also explain the lack of a statistically significant change in FSFI score and only a slightly improved postoperative PISQ-12 score. Interestingly, there was no strong correlation between the PISQ-12 and FSFI, in spite of the fact that both questionnaires are validated measures of female sexual function.
Studies on surgical treatment of pelvic organ prolapse and sexual function have been conducted in several different countries and cultures.33-35 Sexual functions, including expectations for sexual relationships and sexual complaints, vary among cultures.33-35 Accordingly, discrepancies in the results of earlier studies might originate from differences in demographic and clinical characteristics.33-35 Because of these differences, it is difficult to compare outcomes of different studies.
This study was a prospective study that employed specific standardized questionnaires to assess sexual function in a large, well-defined group of women who underwent repair of pelvic organ prolapse. Moreover, our study is the first to evaluate sexual function by both the FSFI and PISQ-12 after surgery for pelvic organ prolapse. Despite these strong points, there are some limitations to our study. Our study population was relatively older (mean age: 65.1 years) and had a high proportion of FSD compared to other studies. In addition, we used FSFI and PISQ-12 translated from English to Korean, the reliability and validity of which have not yet been proven. Two surgeons with native English speaking ability translated these forms in bi-directional way and verified the contents to overcome this weak point.
In conclusion, surgery for pelvic organ prolapse affected some domains of sexual function. However, we were unable to definitively conclude whether repair of pelvic organ prolapse improves sexual function in all patients. In this study, our results for FSFI and PISQ-12 were not correlated with each other, as we had expected, and only menopause affected the scores of both questionnaires. We expect that a more sexually active proportion would have given us better information. Future studies are needed to evaluate sexual function following prolapse surgery using several validated tools in a larger subgroup of women with different demographic and clinical characteristics.
Figures and Tables
ACKNOWLEDGEMENTS
This study was supported by a grant from the Korea Healthcare Technology R&D Project, Ministry for Health, Welfare & Family Affairs, Republic of Korea (A084120).
References
1. Read S, King M, Watson J. Sexual dysfunction in primary medical care: prevalence, characteristics and detection by the general practitioner. J Public Health Med. 1997; 19:387–391.

2. Lemack GE, Zimmern PE. Sexual function after vaginal surgery for stress incontinence: results of a mailed questionnaire. Urology. 2000; 56:223–227.

3. Tok EC, Yasa O, Ertunc D, Savas A, Durukan H, Kanik A. The effect of pelvic organ prolapse on sexual function in a general cohort of women. J Sex Med. 2010; 7:3957–3962.

4. Barber MD, Visco AG, Wyman JF, Fantl JA, Bump RC. Continence Program for Women Research Group. Sexual function in women with urinary incontinence and pelvic organ prolapse. Obstet Gynecol. 2002; 99:281–289.

5. Ozel B, White T, Urwitz-Lane R, Minaglia S. The impact of pelvic organ prolapse on sexual function in women with urinary incontinence. Int Urogynecol J Pelvic Floor Dysfunct. 2006; 17:14–17.

6. Nygaard I, Bradley C, Brandt D. Women's Health Initiative. Pelvic organ prolapse in older women: prevalence and risk factors. Obstet Gynecol. 2004; 104:489–497.

7. Burrows LJ, Meyn LA, Walters MD, Weber AM. Pelvic symptoms in women with pelvic organ prolapse. Obstet Gynecol. 2004; 104(5 Pt 1):982–988.

8. Maher C, Baessler K, Glazener CM, Adams EJ, Hagen S. Surgical management of pelvic organ prolapse in women: a short version Cochrane review. Neurourol Urodyn. 2008; 27:3–12.

9. Olsen AL, Smith VJ, Bergstrom JO, Colling JC, Clark AL. Epidemiology of surgically managed pelvic organ prolapse and urinary incontinence. Obstet Gynecol. 1997; 89:501–506.

10. Weber AM, Walters MD, Piedmonte MR. Sexual function and vaginal anatomy in women before and after surgery for pelvic organ prolapse and urinary incontinence. Am J Obstet Gynecol. 2000; 182:1610–1615.

11. Francis WJ, Jeffcoate TN. Dyspareunia following vaginal operations. J Obstet Gynaecol Br Commonw. 1961; 68:1–10.

12. Haase P, Skibsted L. Influence of operations for stress incontinence and/or genital descensus on sexual life. Acta Obstet Gynecol Scand. 1988; 67:659–661.

13. Bump RC, Mattiasson A, Bø K, Brubaker LP, DeLancey JO, Klarskov P, et al. The standardization of terminology of female pelvic organ prolapse and pelvic floor dysfunction. Am J Obstet Gynecol. 1996; 175:10–17.

14. Wiegel M, Meston C, Rosen R. The female sexual function index (FSFI): cross-validation and development of clinical cutoff scores. J Sex Marital Ther. 2005; 31:1–20.

15. Meston CM. Validation of the Female Sexual Function Index (FSFI) in women with female orgasmic disorder and in women with hypoactive sexual desire disorder. J Sex Marital Ther. 2003; 29:39–46.

16. Rogers RG, Kammerer-Doak D, Villarreal A, Coates K, Qualls C. A new instrument to measure sexual function in women with urinary incontinence or pelvic organ prolapse. Am J Obstet Gynecol. 2001; 184:552–558.

17. Rogers RG, Coates KW, Kammerer-Doak D, Khalsa S, Qualls C. A short form of the Pelvic Organ Prolapse/Urinary Incontinence Sexual Questionnaire (PISQ-12). Int Urogynecol J Pelvic Floor Dysfunct. 2003; 14:164–168.

18. Laumann EO, Paik A, Rosen RC. Sexual dysfunction in the United States: prevalence and predictors. JAMA. 1999; 281:537–544.
19. Bradford A, Meston C. Sexual outcomes and satisfaction with hysterectomy: influence of patient education. J Sex Med. 2007; 4:106–114.

20. Handa VL, Harvey L, Cundiff GW, Siddique SA, Kjerulff KH. Sexual function among women with urinary incontinence and pelvic organ prolapse. Am J Obstet Gynecol. 2004; 191:751–756.

21. Hoda MR, Wagner S, Greco F, Heynemann H, Fornara P. Prospective follow-up of female sexual function after vaginal surgery for pelvic organ prolapse using transobturator mesh implants. J Sex Med. 2011; 8:914–922.

22. Lau HH, Su TH, Su CH, Lee MY, Sun FJ. Short-term impact of tension-free vaginal tape obturator procedure on sexual function in women with stress urinary incontinence. J Sex Med. 2010; 7(4 Pt 1):1578–1584.

23. Achtari C, McKenzie BJ, Hiscock R, Rosamilia A, Schierlitz L, Briggs CA, et al. Anatomical study of the obturator foramen and dorsal nerve of the clitoris and their relationship to minimally invasive slings. Int Urogynecol J Pelvic Floor Dysfunct. 2006; 17:330–334.

24. Baessler K, Maher CF. Mesh augmentation during pelvic-floor reconstructive surgery: risks and benefits. Curr Opin Obstet Gynecol. 2006; 18:560–566.

25. Caruso S, Rugolo S, Bandiera S, Mirabella D, Cavallaro A, Cianci A. Clitoral blood flow changes after surgery for stress urinary incontinence: pilot study on TVT Versus TOT procedures. Urology. 2007; 70:554–557.

26. Xu Y, Song Y, Huang H. Impact of the tension-free vaginal tape obturator procedure on sexual function in women with stress urinary incontinence. Int J Gynaecol Obstet. 2011; 112:187–189.

27. Leone Roberti Maggiore U, Alessandri F, Medica M, Gabelli M, Venturini PL, Ferrero S. Periurethral injection of polyacrylamide hydrogel for the treatment of stress urinary incontinence: the impact on female sexual function. J Sex Med. 2012; 9:3255–3263.

28. Jha S, Ammenbal M, Metwally M. Impact of incontinence surgery on sexual function: a systematic review and meta-analysis. J Sex Med. 2012; 9:34–43.

29. Lose G, Mouritsen L, Nielsen JB. A new bulking agent (polyacrylamide hydrogel) for treating stress urinary incontinence in women. BJU Int. 2006; 98:100–104.

30. Trutnovsky G, Tamussino K, Greimel E, Bjelic-Radisic V. Quality of life after periurethral injection with polyacrylamide hydrogel for stress urinary incontinence. Int Urogynecol J. 2011; 22:353–356.

31. Pauls RN, Silva WA, Rooney CM, Siddighi S, Kleeman SD, Dryfhout V, et al. Sexual function after vaginal surgery for pelvic organ prolapse and urinary incontinence. Am J Obstet Gynecol. 2007; 197:622.

32. Shen T, Song LJ, Xu YM, Gu BJ, Lu LH, Li F. Sexual function and health-related quality of life following anterior vaginal wall surgery for stress urinary incontinence and pelvic organ prolapse. Int J Impot Res. 2011; 23:151–157.

33. Stoutjesdijk JA, Vierhout ME, Spruijt JW, Massolt ET. Does vaginal reconstructive surgery with or without vaginal hysterectomy or trachelectomy improve sexual well being? A prospective follow-up study. Int Urogynecol J Pelvic Floor Dysfunct. 2006; 17:131–135.





 PDF
PDF ePub
ePub Citation
Citation Print
Print







 XML Download
XML Download