Abstract
Purpose
To determine the effects of nonthermal plasma (NTP) induced by helium (He) alone or He plus oxygen (O2) on the generation of reactive oxygen species (ROS) and cell death in anaplastic thyroid cancer cells.
Materials and Methods
NTP was generated in He alone or He plus O2 blowing through a nozzle by applying a high alternating current voltage to the discharge electrodes. Optical emission spectroscopy was used to identify various excited plasma species. The apoptotic effect of NTP on the anaplastic thyroid cancer cell lines, such as HTH83, U-HTH 7, and SW1763, was verified with annexin V/propidium staining and TUNEL assay. ROS formation after NTP treatment was identified with fluorescence-activated cell sorting with DCFDA staining. The mitogen-activated protein kinase pathways and caspase cascade were investigated to evaluate the molecular mechanism involved and cellular targets of plasma.
Results
NTP induced significant apoptosis in all three cancer cell lines. The plasma using He and O2 generated more O2-related species, and increased apoptosis and intracellular ROS formation compared with the plasma using He alone. NTP treatment of SW1763 increased the expression of phosphor-JNK, phosphor-p38, and caspase-3, but not phosphor-ERK. Apoptosis of SW1763 as well as expressions of elevated phosphor-JNK, phosphor-p38, and caspase-3 induced by NTP were effectively inhibited by intracellular ROS scavengers.
The incidence of thyroid cancer, the most common endocrine malignancy and one of the most frequent cancers in women, has gradually increased over the last three decades. In the United States, an estimated 3250 men and 43210 women were diagnosed with thyroid cancer in 2012.1 Fortunately, most thyroid cancers have a good prognosis, and the 5-year survival rate of all patients with thyroid cancer exceeds 95%.1 Among the various types of thyroid cancer, anaplastic thyroid cancer (ATC) is a rare but extremely aggressive form, with a median survival of only 5 months and a 1-year mortality rate exceeding 80%.2,3 Conventional treatment for ATC combines surgery, radiotherapy, and chemotherapy. However, many patients have unresectable disease at the time of diagnosis, and the effect of treatment is very limited. Accordingly, developing a new treatment modality for ATC is necessary to improve the survival of patients with this very lethal disease.
Plasma is a partially ionized medium that contains electrons, ions, radicals, electronically excited molecules, and energetic photons. Recent studies have investigated the use of plasma at room temperature for biological and medical purposes, such as blood coagulation,4 wound healing,5 tissue sterilization,6 and cancer treatment.7 For cancer treatment, studies have revealed that nonthermal plasma can induce the apoptosis of cancer cells in a dose-dependent manner, and that this might be related to DNA damage resulting from the generation of reactive oxygen species (ROS).8,9,10 However, the mechanism involved in the apoptosis is not fully understood, and the optimal gas combination for clinical use is under debate.
In this study, we evaluated whether nonthermal plasma can induce significant apoptosis in ATC cell lines oxygen (O2) concentration-dependently, and investigated the role of ROS in apoptosis and molecular mechanism involved to develop a new potential treatment modality for highly lethal ATC.
The technical specifications of the "torch with spray type" nonthermal plasma system are schematically presented in Fig. 1A and B. We have previously described the characteristics of our plasma system.11,12 With this system, the power supply ranges from 2 to 13 kV, with a mean frequency of 20-30 kHz, depending on the type and amount of gas used. In this study, air (control), helium (He), and O2 were used as carrier gases for the stable and low-temperature plasma process. The temperature of atmospheric-pressure plasma gas can be maintained at around 35℃ even 10 min treatment of 13 kV of plasma treatment; therefore, a temperature effect on the cells can be avoided. To identify various excited plasma species generated by two atmospheric-pressure plasma jet species generated by the plasma jet, optical emission spectroscopy was employed at wavelengths of 270-930 nm (SV2100; K-MAC, Daejeon, Korea).
The human ATC cell lines HTH83, U-HTH 7, and SW1736 were provided by Dr. Yoon Woo Koh (Department of Otorhinolaryngology, Yonsei University College of Medicine, Seoul, Korea). U-HTH 7 cells were cultured in high-glucose Dulbecco's modified Eagle's medium (Gibco/Invitrogen, Carlsbad, CA, USA), whereas HTH83 and SW1736 cells were cultured in RPMI 1640. All cell lines were maintained at 37℃ and 5% CO2 in media supplemented with 10% fetal bovine serum (Gibco/Invitrogen). N-acetyl cysteine (NAC) was purchased from Sigma-Aldrich (St. Louis, MO, USA).
Apoptotic cells were detected using an annexin-V-fluorescein isothiocyanate/propidium iodide (PI) apoptosis detection kit (BD Biosciences, Bedford, MA, USA). Briefly, three milliliter of cell suspension with concentration of 1×105 cells/mL were plated in six-well culture dishes and treated with plasma generated by He with/without O2 followed by incubation for 24 hours. Samples were prepared according to the manufacturer's recommended protocol. Apoptosis was detected using a BDFACSAria III instrument (BD Biosciences) with excitation and emission settings of 488 and 530 nm, respectively.
Cells on the coverslip were treated with nonthermal plasma, then fixed in 4% paraformaldehyde at room temperature for 1 hour and examined for DNA fragmentation using an in situ cell death detection kit (Roche Molecular Biochemicals, Basel, Switzerland) according to the manufacturer's instructions. The stained cells were visualized by fluorescence microscopy (Carl Zeiss, Oberkochen, Germany). The digital images of apoptotic cells were randomly selected.
For measurement of cellular ROS production, SW1736 cells were treated with nonthermal plasma and then treated with 10 µM 5-(and-6)-carboxy-2',7'-dichlorodihydrofluorescein diacetate (carboxy-H2DCFDA) dye (Molecular Probes, Eugene, OR, USA) for 30 min at 37℃. Fluorescence-stained cells (1×104) were then analyzed by flow cytometry.
Cells were lysed in lysis buffer containing 150 mM NaCl, 1.0% NP40, 0.5% sodium deoxycholate, 0.1% SDS, 50 mM Tris (pH 8.0), and protease inhibitor cocktail (Roche) as previously described.13 The following antibodies were used: anti-phospho-JNK, -phopho-p38, -phopho-ERK, -cleaved caspase-3, and -α-tubulin (Cell Signaling Technology, Danvers, MA, USA, 1:1000) for Western blot analysis.
The emission spectrum of He gas plasma revealed the presence of OH (309 nm), second positive system of N2 (391 nm), excited He (667, 706, 728 nm), and weak atomic O2 (777.1, 844 nm). When O2 gas was added to the plasma, some of the peaks changed: the atomic O2 peak (777.1, 844 nm) dominated, and NO (438 nm) and O2+ which were first negative peaks (525, 559 nm) appeared. Therefore, the He and O2 gas mixture plasma generated more O2-related species than did the He gas plasma (Fig. 1C).
A gas-only treatment (He and O2) was used to exclude the gas effects of nonthermal plasma. Gas-only did not show any significant effect on cell viability or apoptosis (Figs. 2 and 3). As shown in Fig. 2, nonthermal plasma treatment induced significant cell death on the ATC cells (SW1736, HTH83, and U-HTH 7). Interestingly, He and O2 gas mixture plasma induced significantly more cell death than the He gas plasma. The SW1736 cells were treated with nonthermal plasma at 2 kV for 1 sec and continuously grown for 24 hours. Nonthermal plasma treatment of SW1736 cells [He only (early, 6.55% and late, 7.5%) with, He plus O2 (early, 8.7% and late, 12.7%)] resulted in significantly higher apoptosis than in the control group (early, 3.65% and late, 4.45%) and gas-only group (early, 4.25% and late, 5.1%) (Fig. 3A): Apoptosis of SW1736 cells was significantly higher in the He-plus-O2 plasma group than in the He-only plasma group (p<0.01) (Fig. 3A). Consistent with the annexin V assay results of SW1736 cells, treatment of HTH83 and U-HTH 7 with nonthermal plasma resulted in increased apoptotic cell death (Fig. 3B and C), respectively.
The TUNEL assay detects DNA ends. Because apoptosis is characterized by DNA fragmentation, increased staining in the nucleus (TUNEL-positive cells) indicates apoptosis. As shown in Fig. 4, no apoptosis was seen in the control or gas-only groups, whereas the plasma using He with/without O2 induced apoptosis in SW1736, HTH83, and U-HTH 7 cells; the apoptosis was greater in the He-plus-O2 plasma group than in the He-only plasma group.
The plasma in the gas-only group did not produce significant amounts of ROS, whereas the plasma in both the He-only plasma group and He-plus-O2 plasma group induced significant ROS formation; the amount of ROS was significantly higher in the He-plus-O2 plasma group. However, the formation of ROS decreased significantly after pretreatment with NAC, an intracellular ROS scavenger (Fig. 5). To identify whether increased ROS formation after plasma treatment resulted in apoptosis in SW1736 cells, we measured the apoptosis rate using Annexin V/PI staining with fluorescence activated cell sorter (FACS). As shown in Fig. 6, plasma-induced apoptosis significantly decreased when ROS formation was inhibited with NAC pretreatment, whereas the plasma treatment using He plus O2 effectively killed SW1763 cells by inducing apoptosis via intracellular ROS formation.
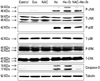
We investigated the mitogen-activated protein kinase (MAPK) pathways and caspase cascade to evaluate the molecular mechanism and cellular targets of plasma. As shown in Fig. 7, Western blotting clearly indicated that plasma treatment increased the expression of phosphor-JNK, phosphor-p38, and caspase-3, but not phosphor-ERK, and these elevated expressions were reduced by adding NAC to the SW1736 cells. These results suggest that the JNK and p38 MAPKs pathways are involved in plasma-induced apoptosis, and that intracellular ROS generated by plasma treatment activated these pathways.
ATC is an undifferentiated epithelial cancer of the thyroid gland and one of the most lethal diseases in humans. The incidence of ATC is only 1.7% of all thyroid cancers in the United States, but the 1-year survival rate is less than 20%.2,3 The most effective treatment of ATC is complete surgical resection, but many patients with ATC have unresectable disease when they are initially diagnosed, and only a small fraction of ATC patients can achieve en bloc resection.14 Accordingly, the effect of conventional combined modality therapy including surgery, chemotherapy, and radiotherapy for ATC is very poor. Many trials have been attempted to develop new treatments for ATC, such as new molecular targeted therapy, anti-tumor immunotherapy, and oncolytic viral therapy; however, there is currently no new therapy that can be recommended over conventional therapy, therefore, there is an urgent need to develop a novel modality for the treatment of ATC.2,15
Nonthermal atmospheric plasma with room temperature, which is different from traditional electrical plasma with high temperature, has recently been developed, and it can expand the clinical applications of plasma to include, for example, the interaction of plasma with living cells. Previous studies have shown that nonthermal plasma can induce cell death, and this might be induced by reactive species, mainly oxygen/hydroxyl radicals and nitric oxide, generated in the plasma or in the tissue when brought into contact with the plasma.16 Cell death induced by plasma can be used in cancer therapy. Since Fridman, et al.17 first observed apoptotic behavior induced by plasma in a melanoma skin cancer cell line, there have been several reports regarding the therapeutic effect of plasma on cancers such as hepatoma, colorectal cancer, lung cancer, cervical cancer, and oral cancer.12,18,19,20,21 We also examined the effect of nonthermal plasma on normal thyroid cells (data not shown). In accordance with the study of Panngom, et al.,20 nonthermal plasma did not significantly decrease the viability of normal cell. However, the mechanism of plasma-induced preferential apoptosis of cancer cells has not yet been fully elucidated.
Our study showed that nonthermal plasma induced apoptosis in all three ATC cell lines, but the control and gas did not. The extent of apoptosis was greater in He-plus-O2 plasma than in He-alone plasma in all three ATC cell lines (Figs. 3 and 4). The results of optical emission spectroscopy showed that He-plus-O2 plasma produced more O2-related species than He-alone plasma, which may be related to the high apoptotic effect. We also observed that ROS were produced after plasma treatment of the ATC cells, but control and gas were not. Apoptosis induced by plasma was inhibited by adding NAC, an intracellular ROS scavenger, indicating that apoptosis induced by plasma is mediated by intracellular ROS formation (Fig. 6). Vandamme, et al.10 reported that treatment with floating electrode dielectric barrier discharge (FE-DBD) plasma induced apoptotic cell death via ROS formation in human glioma cells and colorectal carcinoma cells. Our results are consistent with this earlier report, although we used different cell lines and types of plasma.
We investigated the MAPK pathways and caspase cascade to evaluate the molecular mechanism involved and cellular targets of plasma using Western blot. Our results showed that the expressions of phosphor-JNK, phosphor-p38, and caspase-3, but not phosphor-ERK, were increased after plasma treatment in the ATC cells, and that these elevated expressions were reduced by adding NAC (Fig. 7). These results indicate that among the three major MAPK pathways, JNK and p38 MAPKs are involved in plasma-induced apoptosis, and that intracellular ROS generated by plasma treatment might activate apoptosis signal-regulating kinase 1, which could selectively activate the JNK and p38 MAPK pathways.22,23 Additionally, simultaneously increased expressions of phosphor-JNK, phosphor-p38, and caspase-3 might suggest that apoptosis was mediated in part by mitochondria. Ahn, et al.18 also reported plasma-induced cell death mediated by a mitochondria-dependent pathway in a human cervical cancer cell line. Since, ERK is generally involved in cell growth and proliferation, the finding of no increased expression of phosphor-ERK after plasma treatment is feasible.
In the clinical setting of thyroid cancer, nonthermal plasma with anticancer effect on thyroid cancer cells can be applied immediately after gross removal of tumor in operation field of locally advanced case. Nonthermal plasma could be an alternative adjuvant treatment modality to control locoregional advanced thyroid cancer with tracheal or adjacent soft tissue invasion such as strap muscle and recurrent laryngeal nerve, thereby minimizing complication of airway surgery, irradiation and resection of recurrent laryngeal nerve which have an additional risk of complications.
In this study, there are some limitation in that we should examine the effect of nonthermal plasma treatment on ATC cells in in vivo condition and eventually in the clinical setting. In addition, we are planning more experiments including knockdown and inhibitor study using inhibitors of JNK, and p38, thus conclusively elucidating the association of ROS and JNK/p38 signals with nonthermal plasma induced apoptosis.
In conclusion, this is the first study to evaluate the therapeutic effect of plasma treatment in highly lethal ATC. Our results suggest that plasma treatment induces apoptotic cell death in ATC cell lines, and that the addition of O2 to He during plasma formation improved the efficiency of apoptosis. The main mechanism of apoptosis induced by the plasma is intracellular ROS formation and involves the MAPK pathway. Although many additional studies are required, including in vivo studies, plasma treatment may be a potent alternative therapeutic option for ATC.
Figures and Tables
Fig. 1
(A) Schematic diagram of the plasma torch. (B) Image of the plasma jet with helium (He) and oxygen (O2). (C) Plasma emission spectra with different gases, comparing He versus He+O2 at 2 kV.

Fig. 2
Cell viability assay on ATC cells. (A) SW1736. (B) HTH83. (C) U-HTH 7. Cells were treated with gas only or each type of plasma jets at 2 kV for 1 s. At 24 hours after plasma treatment, cell viability was measured by a MTT assay. The data represent mean±SD of three independent experiments. *p<0.05, **p<0.01, and ***p<0.001 with Student's t-test compared with the control. ATC, anaplastic thyroid cancer; MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide.

Fig. 3
Analysis of the death of ATC cells by annexin V/PI staining. (A) SW1736. (B) HTH83. (C) U-HTH 7. Cells were treated with gas only or plasma jets at 2 kV for 1 s and then incubated for 24 hours, and stained with annexin V-FITC and propidium iodide (PI). Apoptosis was detected using a FACS system. The data represent mean±SD of three independent experiments. *p<0.05, **p<0.01, and ***p<0.001 with Student's t-test compared with the control. FITC, fluorescein isothiocyanate; ATC, anaplastic thyroid cancer; FACS, fluorescence activated cell sorter.

Fig. 4
Analysis of ATC cell death using TUNEL staining. (A) SW1736. (B) HTH83. (C) U-HTH 7. The TUNEL analysis was performed for DNA fragmentation using an in situ cell death detection kit under a light microscope; the digital images of apoptotic cells were selected randomly. Scale bars denote 50 µm. ATC, anaplastic thyroid cancer.
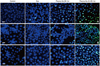
Fig. 5
Measurement of ROS production by nonthermal plasma. After plasma treatment, the SW1736 cells were treated with carboxy-H2DCFDA dye and then analyzed by flow cytometry. The FACS data from three independent experiments were quantified. The data represent mean±SD of three independent experiments. *p<0.05, **p<0.01 with Student's t-test compared with the control. NS, not significant. NAC, N-acetyl cysteine; ROS, reactive oxygen species; FITC, fluorescein isothiocyanate; FACS, fluorescence activated cell sorter.

Fig. 6
Evaluation of cell death due to ROS generated by nonthermal plasma using annexin V/PI staining. The SW1736 cells were treated with gas only or plasma jets at 2 kV for 1 s and then incubated for 24 hours, and stained with annexin V-FITC and propidium iodide (PI). Apoptosis was detected using a FACS Canto system. The data represent mean±SD of three independent experiments. *p<0.05, **p<0.01, and ***p<0.001 compared with the control using Student's t-test. NS, not significant; ROS, reactive oxygen species; FITC, fluorescein isothiocyanate; FACS, fluorescence activated cell sorter.

Fig. 7
Western blot analysis. Cells were suspended in RIPA buffer supplemented with PhosSTOP and cOmplete Mini EDTA-free. The proteins from SW1736 cells were electrotransferred to Immobilon-P membranes. Specific proteins were detected with an ECL Western Blotting Kit. NAC, N-acetyl cysteine; EDTA, ethylenediaminetetraacetic acid; ECL-enhanced chemiluminescent.
ACKNOWLEDGEMENTS
This research was supported by the Bio & Medical Technology Development Program of the National Research Foundation (NRF), funded by the Korean government (MEST; 2012M3A9B2052870).
References
1. Johnston LE, Tran Cao HS, Chang DC, Bouvet M. Sociodemographic Predictors of Survival in Differentiated Thyroid Cancer: Results from the SEER Database. ISRN Endocrinol. 2012; 2012:384707.
2. Smallridge RC, Copland JA. Anaplastic thyroid carcinoma: pathogenesis and emerging therapies. Clin Oncol (R Coll Radiol). 2010; 22:486–497.

3. Kebebew E, Greenspan FS, Clark OH, Woeber KA, Grunwell J. Extent of disease and practice patterns for medullary thyroid cancer. J Am Coll Surg. 2005; 200:890–896.

4. Kalghatgi SU, Fridman G, Cooper M, Nagaraj G, Peddinghaus M, Balasubramanian M, et al. Mechanism of blood coagulation by nonthermal atmospheric pressure dielectric barrier discharge plasma. IEEE Trans Plasma Sci. 2007; 35:1559–1566.

5. Isbary G, Morfill G, Schmidt HU, Georgi M, Ramrath K, Heinlin J, et al. A first prospective randomized controlled trial to decrease bacterial load using cold atmospheric argon plasma on chronic wounds in patients. Br J Dermatol. 2010; 163:78–82.

6. Fridman G, Friedman G, Gutsol A, Shekhter AB, Vasilets VN, Fridman A. Applied Plasma Medicine. Plasma Process Polym. 2008; 5:503–533.

7. Vandamme M, Robert E, Dozias S, Sobilo J, Lerondel S, Le Pape A, et al. Response of Human Glioma U87 Xenografted on Mice to Non Thermal Plasma Treatment. Plasma Med. 2011; 1:27–43.

8. Olofsson BA, Kelly CM, Kim J, Hornsby SM, Azizkhan-Clifford J. Phosphorylation of Sp1 in response to DNA damage by ataxia telangiectasia-mutated kinase. Mol Cancer Res. 2007; 5:1319–1330.

9. Kalghatgi S, Kelly CM, Cerchar E, Torabi B, Alekseev O, Fridman A, et al. Effects of non-thermal plasma on mammalian cells. PLoS One. 2011; 6:e16270.

10. Vandamme M, Robert E, Lerondel S, Sarron V, Ries D, Dozias S, et al. ROS implication in a new antitumor strategy based on non-thermal plasma. Int J Cancer. 2012; 130:2185–2194.

11. Kim CH, Kwon S, Bahn JH, Lee K, Jun SI, Rack PD, et al. Effects of atmospheric nonthermal plasma on invasion of colorectal cancer cells. Appl Phys Lett. 2010; 96:243701.

12. Kim CH, Bahn JH, Lee SH, Kim GY, Jun SI, Lee K, et al. Induction of cell growth arrest by atmospheric non-thermal plasma in colorectal cancer cells. J Biotechnol. 2010; 150:530–538.

13. Lee BS, Kang SU, Hwang HS, Kim YS, Sung ES, Shin YS, et al. An agonistic antibody to human death receptor 4 induces apoptotic cell death in head and neck cancer cells through mitochondrial ROS generation. Cancer Lett. 2012; 322:45–57.

14. Akaishi J, Sugino K, Kitagawa W, Nagahama M, Kameyama K, Shimizu K, et al. Prognostic factors and treatment outcomes of 100 cases of anaplastic thyroid carcinoma. Thyroid. 2011; 21:1183–1189.

15. Deshpande HA, Gettinger SN, Sosa JA. Novel chemotherapy options for advanced thyroid tumors: small molecules offer great hope. Curr Opin Oncol. 2008; 20:19–24.

16. Chen N, Garner AL, Chen G, Jing Y, Deng Y, Swanson RJ, et al. Nanosecond electric pulses penetrate the nucleus and enhance speckle formation. Biochem Biophys Res Commun. 2007; 364:220–225.

17. Fridman G, Shereshevsky A, Jost MM, Brooks AD, Fridman A, Gutsol A, et al. Floating Electrode Dielectric Barrier Discharge Plasma in Air Promoting Apoptotic Behavior in Melanoma Skin Cancer Cell Lines. Plasma Chem Plasma Process. 2007; 27:163–176.

18. Ahn HJ, Kim KI, Kim G, Moon E, Yang SS, Lee JS. Atmospheric-pressure plasma jet induces apoptosis involving mitochondria via generation of free radicals. PLoS One. 2011; 6:e28154.

19. Zhang X, Li M, Zhou R, Feng K, Yang S. Ablation of liver cancer cells in vitro by a plasma needle. Appl Phys Lett. 2008; 93:021502–021503.
20. Panngom K, Baik KY, Nam MK, Han JH, Rhim H, Choi EH. Preferential killing of human lung cancer cell lines with mitochondrial dysfunction by nonthermal dielectric barrier discharge plasma. Cell Death Dis. 2013; 4:e642.

21. Han X, Klas M, Liu Y, Stack MS, Ptasinska S. DNA damage in oral cancer cells induced by nitrogen atmospheric pressure plasma jet. Appl Phys Lett. 2013; 102:233703.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download