Abstract
Purpose
The aim of this study was to investigate the effect of epidural dexamethasone on analgesia and cytosolic phospholipase A2 (cPLA2) expression in the spinal cord in a rat formalin test.
Materials and Methods
Epidural dexamethasone injection was performed to Sprague-Dawley rats with a 25 gauge needle under fluoroscopy. Following the epidural injection, a formalin induced pain behavior test was performed. Next, the spinal cords corresponding to L4 dorsal root ganglion was extracted to observe the cPLA2 expression.
Results
There were no differences in pain response during phase I among the groups. The phase II pain response in 300 µg of epidural dexamethasone group decreased as compared to control, 30 µg of epidural dexamethasone, 100 µg of epidural dexamethasone, and 300 µg of systemic dexamethasone groups. The expression of cPLA2 decreased in Rexed laminae I-II in 300 µg of the epidural dexamethasone group compared with the ones in the control group.
Conclusion
Taken together, these results suggest that 300 µg of epidural dexamethasone has an attenuating effect on the peripheral inflammatory tissue injury induced hyperalgesia and this effect is mediated through the inhibition of intraspinal cPLA2 expression and the primary site of action is the laminae I-II of the spinal cord.
Managing postoperative pain relieves suffering and leads to earlier mobilization, shortened hospital stay, reduced hospital costs, and increased patient satisfaction.1 Various adjuvants have been used to enhance analgesia, prolong the analgesic duration, and reduce opioid requirement and their side effects. Effective postoperative pain management involves a multimodal approach and the use of various drugs with different mechanisms of action.
Tissue injury and inflammation cause activation of cytosolic phospholipase A2 (cPLA2) which yields the release of arachidonic acids from intracellular membrane phospholipids.2 The released arachidonic acids are processed by cyclooxygenase to generate eicosanoids including prostaglandins, thromboxanes, prostacyclins, and leukotrienes. Glucocorticoids have anti-inflammatory effects arising from the inhibition of cPLA2 expression and inhibition of NF-κB mediated transcription of various cytokines in the injured tissues.3,4 An epidural steroid injection is used for the treatment of patients with spinal stenosis and disc herniation to reduce the local inflammation and edema around the nerve roots or spinal cord.5
Also, peripheral tissue injury could activate the function of cPLA2 in the spinal cord. Noxious peripheral stimulation causes a release of inflammatory mediators and sustains activation of the nociceptive afferent fiber. Sustained activity of the afferent fiber causes an increased release of neurotransmitters including glutamate, substance P, and calcitonin gene related peptide in the spinal cord dorsal horn.6 These released neurotransmitters interact with N-methyl-D-aspartate and neurokinin receptor and lead to an increase of the intracellular calcium ion concentration.7 The increased intracellular calcium ion subsequently activates cPLA2 expression.8 Activated cPLA2 results arachidonic acid from phospholipids and resulted arachidonic acid yields prostaglandin synthesis in the spinal cord those are related to peripheral tissue injury induced hyperalgesia.9,10
Recently, several groups have reported the analgesic effect of preoperative epidural dexamethasone treatment on postoperative pain.11,12,13 However, the mechanism involved in the analgesic effect as a result of epidural dexamethasone in the peripheral tissue injury has not been examined. We hypothesized that the treatment with epidural dexamethasone could inhibit the expression of cPLA2 in the spinal cord, and it may lead to a reduction in the hyperalgesia produced from the peripheral tissue injury.
Therefore, the aim of present study was to examine: 1) the analgesic effect of epidural dexamethasone at different doses, and 2) the effect of epidural dexamethasone on cPLA2 expression in the spinal cord in a rat formalin test.
The experiments within our study were approved in accordance with the Institutional Animal Care and Use Committee in Korea University (Approval No. KUIACUC-2011-154). Male Sprague-Dawley rats (Central Laboratory Animal, Seoul, Korea) weighing 250-300 g were used in the experiments. All study protocols were performed using conduct guidelines established by the National Academy of Sciences' Institute for Laboratory Animal Research. Before the experiments, the animals were given unlimited access to food and water. The room lights were maintained on a light-dark cycle of 12 hour periods. Room temperature was maintained at 24-26℃.
The animals were anesthetized in a transparent acryl box with 5% sevoflurane and oxygen flow. Anesthesia was maintained with a 2% sevoflurane mask during the epidural procedure. To enlarge the lumbar interlaminar space, a 2.5 inch elastic band roll was used as an abdomen pillow. We performed the epidural drug injection with 25 gauge Quincke-type needle (Kovax-Needle®, Korea Vaccine Co., Seoul, Korea) under fluoroscopic guidance with C-arm apparatus (Ziehm Exposcop 8000, Ziehm Imaging, Hesse, Germany). The needle was bent to the symmetrical direction of the bevel (about 30 degree at the needle hub) to identify the direction of the needle bevel (Fig. 1). The needle was inserted percutaneously into the L5/6 or L6/S1 interlaminar space (Fig. 2A). After contact with the dense ligamentum flavum, we rotated the direction of the needle so that the bevel became parallel with that of dura mater (Fig. 2B). We cautiously advanced the needle to the epidural space with the loss of resistance technique with saline in a 1 mL disposable plastic syringe (Kovax-Syringe®, Korea Vaccine Co., Seoul, Korea) (Fig. 2C) and confirmed whether the needle tip is in the epidural space using contrast dye (Fig. 2D). The entire lumbar and lower thoracic epidural space was filled with 0.2 mL of contrast dye (Fig. 2E), which is identical to the volume of epidural drugs used in our study. After the epidural injection, the contrast dye was washed out (Fig. 2F). Anteroposterior and lateral radiographs after contrast dye injection were obtained to confirm the extent and dispersal pattern within the epidural space (Fig. 3).
The animals were tested before and after epidural drug injection using placing and stepping response, righting reflex, and observation of posture and gait.14 To avoid any unintentional or undetectable intrathecal injection or neural damage caused by the procedure, we excluded rats that exhibited abnormalities in righting response, placing or stepping, gait or posture. Also, we excluded rats that exhibited sudden movement of the hind paw or tail during the procedure.
The six experimental groups were as followings (n=10 per group). The control group remained with no epidural intervention. The vehicle group received an epidural treatment with the 0.2 mL of normal saline into the epidural space. Epidural dexamethasone (dexamethasone crystalline, Sigma-Aldrich Co., St. Louis, MO, USA) was given as a 30 µg, 100 µg, or 300 µg doses in D30, D100, and D300 groups respectively. For injections into the epidural dexamethasone groups, we prepared dexamethasone solutions as 0.15, 0.5, and 1.5 µg/µL concentrations and injected 0.2 mL into each group. To identify whether effects are peripheral or spinal cord mediated, we gave 300 µg of intraperitoneal dexamethasone to the S300 group.
A half hour following the epidural treatment, 50 µL of 2% formalin solution (37% formaldehyde, Sigma-Aldrich Co., St. Louis, MO, USA) was injected subcutaneously into the dorsal surface of the right hind paw using a 26 gauge needle. After formalin injection, the rat was put in a transparent acryl box and was observed for pain behaviors. Flinching was defined as a rapid and brief withdrawal or flexing of the injected paw. We counted the number of flinches as pain behaviors and recorded the totals in five minute intervals up to one hour.15,16 The total sum of flinches during the initial 10 minutes (phase I: 0-10 min) and following 50 minutes (phase II: 11-60 min) was calculated. Formalin induced pain behavior was assessed by one observer who did not know the type of groups.
The cPLA2 expression in the spinal cord an hour after the pain behavior test in the naive (no epidural intervention and no formalin test), control (no epidural intervention with formalin test), and D300 (epidural dexamethasone 300 µg with formalin test) groups were compared (n=10 per group). Animals were sacrificed an hour after the pain behavior test using ketamine 50 mg/kg and xylazine 5 mg/kg given intraperitoneally and 300-400 mL of phosphate buffer saline (PBS) was perfused from the left ventricle and drained to the right atrium. Next, we fixed the rat tissue with a 4% paraformaldehyde/PBS solution and harvested the spinal cord section corresponding to the L4 nerve root that innervates the hind paw dorsum through muscle dissection and laminectomy.
After fixation of the extracted cord segment with 4% paraformaldehyde/PBS for 24 hours, the tissue was sectioned into 30-40 µm thickness using a sliding microtome (Leica SM2010®, Leica, Wetzlar, Germany). To remove traces of fixatives, sliced tissue sections were washed with PBS in a 24-well plate (24-well Cell Culture Plate, Becton Dickinson and Company, Sparks, MD, USA) in a floating state. To block nonspecific binding of antibodies, tissue sections were pre-incubated with 10% normal goat serum (normal goat serum, Vector Laboratories, Inc., Burlingame, CA, USA) for an hour on a shaker (150 rpm, 4℃). Primary antibody reaction of the tissue sections was performed with a 1:500 dilution of rabbit anti-cPLA2 antibody (LS-C17270, LifeSpan BioSciences Inc., Seattle, WA, USA) for 48 hours. Optimal antibody concentrations were determined following protocols from a previously published report as well as preliminary experiments.9,17 Following the primary antibody incubation, tissue sections were permeabilized and rinsed three times using 0.5 M PBS/0.3% Triton X-100/ in 1% goat serum. The secondary antibody reaction was performed using a 0.5% biotinylated anti-rabbit IgG antibody (BA-1000, Vector Laboratories, Inc., Burlingame, CA, USA)/0.3% Triton X-100/0.5 M PBS, and incubated for one hour. Following incubation, the tissue sections were reacted for one hour with PBS and a 0.5% avidin-biotin peroxidase complex (avidin/biotin blocking kit, Vector Laboratories, Inc., Burlin-game, CA, USA)/0.3% Triton X-100/PBS, and followed by an additional PBS rinse. The reaction on tissue sections was visualized by washing with 2.5 mol/L Acetate buffer (pH 5.0) and 0.2% 3-amino-9-ethylcarbazole (AEC) in N,N-dimethylformamide, and catalyzing of AEC with 3% H2O2 and AEC/Acetate for 10 minutes. The AEC chromogen had been changed to red color. Next, the tissue sections were counterstained with hematoxylin for 2 seconds and rinsed in gently running tap water for 5 minutes. Tissue sections were mounted with glycerol jelly and coverslipped for microscopic observation. Images were taken at 40× and 400× magnifications and with a microscope (Olympus BX51 Research Microscope, Olympus, Tokyo, Japan) and camera (Olympus DP72 Cooled Camera, Olympus, Tokyo, Japan).
To determine which part of the spinal cord dorsal horn was involved in cPLA2 expression, five sections from each rat were randomly selected and we analyzed the images with image quantification program (ImageJ, National Institutes of Health, Bethesda, MD, USA).18 The threshold was selected at the stained cytoplasmic spots that had reacted with the anti-cPLA2 antibody. We established antibody specific immunoreactivity (ASI) as the area exhibited a stronger intensity than the threshold, and compared the ASI in three Rexed laminae levels (I-II, III-IV, V-VI) among the naive, control, and D300 groups.
The numbers of flinches were expressed as mean±standard error of mean (SEM), and areas of ASI were expressed as median and interquartile range (25% and 75% of IQR). Statistical analysis was performed using SPSS program (SPSS 18.0, SPSS Inc., Chicago, IL, USA). One way analysis of variance followed by Dunnett post-hoc test was used for pain behavior comparisons, and Kruskal-Wallis test followed by Mann-Whitney post-hoc test for ASI comparison. p values less than 0.05 were considered statistically significant.
Results demonstrated that rats exhibited a biphasic response to pain. The phase I pain response lasted for first 10 minutes, and after a subsequent late phase II pain response appeared and persisted up to 60 minutes. There were no differences in phase I pain response in all groups. There was no difference in phase I and II pain response between the control and D30 groups (Fig. 4). However, the phase II pain response of D100 and D300 groups demonstrated a significant decrease as compared to control and D30 groups (*p<0.005 vs. control, †p<0.005 vs. D30) (Fig. 4). The phase II pain response of D300 group showed a significant decrease compared to D100 group (‡p<0.05 vs. D100) (Fig. 4).
We observed cytoplasmic staining for cPLA2 in the I-VI layers of Rexed laminae in naive, control, and D300 groups at 40× and 400× magnifications (Fig. 6). In contrast to naive group, the cPLA2 expressions were observed clearly throughout the entire Rexed laminae in control group (Fig. 6A and B). In D300 group, the cPLA2 expressions in the laminae I-II were similar to that of naive group; however, the cPLA2 expressions in the laminar III-IV and V-VI were similar to that of control group (Fig. 6C). Fig. 6D, E, and F are the 10× magnified image of each quadrangle in naive, control, and D300 groups. The naive and D300 groups showed dimmer staining of the cPLA2 as compared to control group in laminae I-II (Fig. 6D, E, and F).
Quantitative analysis of our resulting images using ImageJ demonstrated that the ASI of the laminae I-II in D300 and naive groups were significantly decreased in comparison to control group (*p<0.001 vs. naive, †p<0.001 vs. D300) (Fig. 7). However, the ASI of the laminae III-IV and V-VI in control and D300 groups were significantly increased in comparison to naive group (*p<0.001 vs. naive) (Fig. 7).
We observed a selective dose-dependent decrease in the phase II pain response in the epidural dexamethasone group and our results demonstrating that this decrease was not observed in the control and systemic dexamethasone groups. Also, we found that the administration of 300 µg of epidural dexamethasone inhibited cPLA2 expression in the laminae I-II of the spinal cord. These findings support our hypothesis that the epidural dexamethasone inhibits the expression of intraspinal cPLA2 and results in a decrease of hyperalgesia produced from the peripheral inflammatory tissue injury.
Injection in a rat with formalin causes a typical biphasic pain response. The phase I pain response occurs by primary afferent fiber activation due to the peripheral noxious stimulation. The progression of peripheral inflammatory reaction sustains the C fiber activity and results functional change in the spinal cord dorsal horn and produces hyperexcitability which affects the phase II pain response.19 This pain model has been used as an experimental model of central sensitization to pain and recommended as a useful technique for evaluating the analgesic effects of various agents.15,16,19
To the best of our knowledge, this is the first report showed that epidural dexamethasone influences the intraspinal cPLA2 expression in laminae I-II after a peripheral tissue injury. Spinal disposition of opioids after epidural administration has been studied, and the mechanism is explained as diffusion.20,21 Although, the effect of epidurally administered steroid on the spinal cord has not been well studied, Hayashi, et al.22 showed that the epidural steroid treatment decreased hyperalgesia and decreased the staining intensity of substance-P and calcitonin gene related peptide in laminar I and II in a rat model of radiculopathy. Rexed laminae I-II is a superficial layer of the spinal cord dorsal horn that receives projections from the afferent fibers.23 Dexamethasone in the epidural space could diffuse more easily into the superficial layers than into the deep layers of the spinal cord. In our experiments, 300 µg of epidural dexamethasone inhibited expression of cPLA2 in the laminae I-II. However, cPLA2 expression in the control group was observed throughout the entire Rexed laminae. This suggests that the main site of action in the spinal cord of epidurally administered dexamethasone is laminae I-II.
Nociceptive primary A-delta fibers synapse in laminae I-II and V, and primary C fibers synapse in laminae I-II.23 In a rat formalin test, the functional changes in the dorsal horn neurons were initiated by C fiber activation.19 In a rat with complete Freund's adjuvant (CFA) induced inflammation, A-beta fibers sprout into the substantia gelatinosa (laminae II) from their original location (laminae III-V) and contribute to the development of secondary hyperalgesia and allodynia.24 Laminae I-II has been established as the location for expression of c-fos (known as a pain marker protein) as a direct result of formalin injection or thermal stimulation in a rat.25 Extracellular signal-regulated kinase (ERK) is normally activated by high-threshold noxious stimulation and mediates mechanical allodynia and spreading of pain after inflammation. However, CFA induced inflammation in a rat, the light touch induced ERK in laminae I-II and caused allodynia.26 These reports suggest that the laminae I-II is an important area where sensitization occurs, and emphasize the significance of inhibition of cPLA2 expression in laminae I-II in our experiment.
Among the PLA2 isoforms, recent studies have focused on the group IV calcium-dependent cytosolic cPLA2, the group II small molecule secretory sPLA2, and the group VI calcium-independent iPLA2.27 The enzyme cPLA2 is ubiquitously present in most mammalian cells and tissues. The cPLA2 in the spinal cord is considered as an important mediator of hyperalgesia after tissue injury and inflammation. Under normal conditions, intraspinal cPLA2 maintains normal cellular function. However, under pathologic conditions, it accumulates prostaglandins and induces central sensitization.10,17 In a rat formalin test, intrathecally administered cPLA2 antagonist decreased phase II pain response, prostaglandin E2 release, and group IVA cPLA2 expression in the spinal cord.9,28 Annexin A1 has been reported that modulating nociceptive processing at the spinal level, by reducing synthesis of prostaglandin E2 by modulating cPLA2 in a mouse.10 This annexin A1 is the second messenger that mediates the anti-inflammatory actions of dexamethasone.29 These reports are consistent with our results that the inhibition of cPLA2 in the spinal cord with epidural dexamethasone was accompanied by a decrease in peripheral inflammatory tissue injury induced hyperalgesia.
In our experiments, there was no effect of epidural dexamethasone on phase I pain response. It means that the epidural dexamethasone does not have the direct effect on acute afferent fiber nociceptive processing. It is same as the result of behavior test that inhibition of intraspinal group IVA cPLA2 had no effect on acute pain sensation during phase I, but alleviated the formalin induced pain response during phase II.9,28 Same as our results, Abram, et al.14 reported that repeated intrathecal administration of 250 µg of triamcinolone reduced phase II pain response, but had no effect on phase I pain response in a rat formalin test.
In our experiments, 300 µg of epidural dexamethasone reduced the phase II pain response, but 300 µg of intraperitoneal dexamethasone did not. This suggests that the dose of 300 µg of dexamethasone was sufficient to block the pain response via the epidural administration, but not via the systemic administration. It means that the analgesic effect of dexamethasone in our experiment mainly comes from the spinal cord mediated, not from the peripheral tissue mediated.
As no previous experiment has evaluated the efficacy of epidural dexamethasone in a rat, we chose the dose of 30 µg in D30 group which is comparable to 5 mg to human when the dose and weight of the subjects are compared.11,12,13 Also, we used two higher doses of 100 µg and 300 µg of epidural dexamethasone, because the body surface area of human is larger than that of rat.30 We observed the dose-dependent decrease in phase II pain response as epidural dexamethasone dose increases from 30 µg to 100 µg and 300 µg. Although it is not reasonable to determine the clinical dose by scaling the body weight between the rat and human, our results imply that a higher dose of epidural dexamethasone might induce superior analgesia in the human clinical settings. However, potential complications including immune-suppression and issues with wound healing problem will need to be considered when determining the dose of epidural dexamethasone.
To the best of our knowledge, this is the first study using an epidural drug injection in rats without any operative procedure. Kim, et al.31 used epidurography to confirm whether the surgically inserted epidural catheter was correctly positioned. In our study, it was possible to inject the target drug into the epidural space correctly using fluoroscopic guidance and confirmation. Previous studies of epidural medications in rats have been done by epidural catheter insertion which required surgical procedures.31,32 However, surgical intervention promotes pain, thereby conflicting and interfering with the analysis of pain in the study. In our study, we could eliminate the effect of this unnecessary surgical intervention using the injection technique described.
It has been reported that central sensitization contribute to postoperative pain and hyperalgesia.33,34 In this respect, epidural dexamethasone could be a rational method in managing postoperative pain. To increase the effectiveness of epidural dexamethasone, it is considerable to combine with local anesthetics that can block primary afferent fiber activity, and analgesics that can act on the deep layers of the spinal cord dorsal horn.
In conclusion, the phase II pain response in 300 µg of epidural dexamethasone group decreased as compared to other groups. The expression of cPLA2 in Rexed laminae I-II in 300 µg of epidural dexamethasone group decreased as compared with the ones in control group. Taken together, these results suggest that 300 µg of epidural dexamethasone has an attenuating effect on the peripheral inflammatory tissue injury induced hyperalgesia and the effect is mediated through the inhibition of cPLA2 and the primary site of action is the laminae I-II in the spinal cord.
Figures and Tables
 | Fig. 2Confirmation of an epidural injection using contrast dye under fluoroscopic guidance. (A) The needle was directed to the ligamentum flavum. (B) The bevel of the needle was turned parallel to the dura. (C) The needle approached the epidural space using the loss of resistance technique. (D) Contrast dye (arrows) was injected to confirm the position of the needle. (E) The epidural space was filled with contrast dye (arrows). (F) After the epidural drug injection, the contrast dye was washed out. |
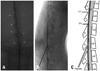 | Fig. 3Anteroposterior (A) and lateral (B) radiographs after contrast dye injection. The needle was positioned centrally within the dorsal epidural space. The contrast material was seen circumferentially within the epidural space and along the proximal nerve sheaths (arrows). (C) Schematic representation of lateral radiograph depicts needle placement and orientation relative to structures. ES, epidural space; IT, intrathecal space; LF, ligamentum flavum; S, spinous process; VB, vertebral body. |
 | Fig. 4Dose-dependent response of epidural dexamethasone on formalin induced pain behaviors. (A) Time course of pain behaviors. The numbers of flinches were used as an index of pain response. (B) Bar graphs show the total sum of flinches during phase I (0-10 min) and phase II (11-60 min). *p<0.005 vs. control, †p<0.005 vs. D30, ‡p<0.05 vs. D100. Symbols and error bars represent the mean±SEM (n=10). SEM, standard error of mean. |
 | Fig. 5Effect of epidural and systemic dexamethasone on formalin induced pain behaviors. (A) Time course of pain behaviors. The numbers of flinches were used as an index of pain response. (B) Bar graphs show the total sum of flinches during phase I (0-10 min) and phase II (11-60 min). *p<0.001 vs. control, †p<0.001 vs. vehicle, ‡p<0.005 vs. S300. Symbols and error bars represent the mean±SEM (n=10). SEM, standard error of mean. |
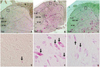 | Fig. 6Microscopic view of cytoplasmic staining for cPLA2 in the ipsilateral spinal cord dorsal horn. Three sections shown within the dotted lines represented the ipsilateral Rexed laminae I-II, III-IV, V-VI each. 40× magnified image of dorsal horn in naive (A), control (B), and D300 groups (C). (D, E, and F) are the 10× magnified image of each quadrangle in naive (A), control (B), and D300 groups (C). Scale bar=100 µm (A, B, and C). cPLA2, cytosolic phospholipase A2 (arrows). |
Notes
References
1. Shang AB, Gan TJ. Optimising postoperative pain management in the ambulatory patient. Drugs. 2003; 63:855–867.

2. Qi HY, Daniels MP, Liu Y, Chen LY, Alsaaty S, Levine SJ, et al. A cytosolic phospholipase A2-initiated lipid mediator pathway induces autophagy in macrophages. J Immunol. 2011; 187:5286–5292.

3. Rhen T, Cidlowski JA. Antiinflammatory action of glucocorticoids--new mechanisms for old drugs. N Engl J Med. 2005; 353:1711–1723.

4. De Bosscher K, Vanden Berghe W, Haegeman G. The interplay between the glucocorticoid receptor and nuclear factor-kappaB or activator protein-1: molecular mechanisms for gene repression. Endocr Rev. 2003; 24:488–522.

5. Abdi S, Datta S, Trescot AM, Schultz DM, Adlaka R, Atluri SL, et al. Epidural steroids in the management of chronic spinal pain: a systematic review. Pain Physician. 2007; 10:185–212.
6. Sluka KA, Dougherty PM, Sorkin LS, Willis WD, Westlund KN. Neural changes in acute arthritis in monkeys. III. Changes in substance P, calcitonin gene-related peptide and glutamate in the dorsal horn of the spinal cord. Brain Res Brain Res Rev. 1992; 17:29–38.

8. Evans JH, Spencer DM, Zweifach A, Leslie CC. Intracellular calcium signals regulating cytosolic phospholipase A2 translocation to internal membranes. J Biol Chem. 2001; 276:30150–30160.

9. Kim DH, Fitzsimmons B, Hefferan MP, Svensson CI, Wancewicz E, Monia BP, et al. Inhibition of spinal cytosolic phospholipase A(2) expression by an antisense oligonucleotide attenuates tissue injury-induced hyperalgesia. Neuroscience. 2008; 154:1077–1087.

10. Ayoub SS, Yazid S, Flower RJ. Increased susceptibility of annexin-A1 null mice to nociceptive pain is indicative of a spinal antinociceptive action of annexin-A1. Br J Pharmacol. 2008; 154:1135–1142.

11. Thomas S, Beevi S. Epidural dexamethasone reduces postoperative pain and analgesic requirements. Can J Anaesth. 2006; 53:899–905.

12. Khafagy HF, Refaat AI, El-Sabae HH, Youssif MA. Efficacy of epidural dexamethasone versus fentanyl on postoperative analgesia. J Anesth. 2010; 24:531–536.

13. Jo YY, Yoo JH, Kim HJ, Kil HK. The effect of epidural administration of dexamethasone on postoperative pain: a randomized controlled study in radical subtotal gastrectomy. Korean J Anesthesiol. 2011; 61:233–237.

14. Abram SE, Marsala M, Yaksh TL. Analgesic and neurotoxic effects of intrathecal corticosteroids in rats. Anesthesiology. 1994; 81:1198–1205.

15. Yoon MH, Park KD, Lee HG, Kim WM, An TH, Kim YO, et al. Additive antinociception between intrathecal sildenafil and morphine in the rat formalin test. J Korean Med Sci. 2008; 23:1033–1038.

16. Kim MJ, Lee WH, Ko YK, Hong BH. Antinociceptive drug interaction between intrathecal vitamin E and gabapentin in the rat formalin test. Korean J Anesthesiol. 2012; 63:447–453.

17. Ong WY, Horrocks LA, Farooqui AA. Immunocytochemical localization of cPLA2 in rat and monkey spinal cord. J Mol Neurosci. 1999; 12:123–130.

18. Papadopulos F, Spinelli M, Valente S, Foroni L, Orrico C, Alviano F, et al. Common tasks in microscopic and ultrastructural image analysis using ImageJ. Ultrastruct Pathol. 2007; 31:401–407.

19. Tjølsen A, Berge OG, Hunskaar S, Rosland JH, Hole K. The formalin test: an evaluation of the method. Pain. 1992; 51:5–17.

20. Bernards CM, Hill HF. Physical and chemical properties of drug molecules governing their diffusion through the spinal meninges. Anesthesiology. 1992; 77:750–756.

21. Bernards CM, Sorkin LS. Radicular artery blood flow does not redistribute fentanyl from the epidural space to the spinal cord. Anesthesiology. 1994; 80:872–878.

22. Hayashi N, Weinstein JN, Meller ST, Lee HM, Spratt KF, Gebhart GF. The effect of epidural injection of betamethasone or bupivacaine in a rat model of lumbar radiculopathy. Spine (Phila Pa 1976). 1998; 23:877–885.

23. Kelly DJ, Ahmad M, Brull SJ. Preemptive analgesia I: physiological pathways and pharmacological modalities. Can J Anaesth. 2001; 48:1000–1010.

24. Nakatsuka T, Park JS, Kumamoto E, Tamaki T, Yoshimura M. Plastic changes in sensory inputs to rat substantia gelatinosa neurons following peripheral inflammation. Pain. 1999; 82:39–47.

25. Lima D, Avelino A, Coimbra A. Differential activation of c-fos in spinal neurones by distinct classes of noxious stimuli. Neuroreport. 1993; 4:747–750.

26. Gao YJ, Ji RR. Light touch induces ERK activation in superficial dorsal horn neurons after inflammation: involvement of spinal astrocytes and JNK signaling in touch-evoked central sensitization and mechanical allodynia. J Neurochem. 2010; 115:505–514.

27. Sun GY, Shelat PB, Jensen MB, He Y, Sun AY, Simonyi A. Phospholipases A2 and inflammatory responses in the central nervous system. Neuromolecular Med. 2010; 12:133–148.

28. Lucas KK, Svensson CI, Hua XY, Yaksh TL, Dennis EA. Spinal phospholipase A2 in inflammatory hyperalgesia: role of group IVA cPLA2. Br J Pharmacol. 2005; 144:940–952.

29. Brooks AC, Rickards KJ, Cunningham FM. Modulation of equine neutrophil adherence and migration by the annexin-1 derived N-terminal peptide, Ac2-26. Vet Immunol Immunopathol. 2012; 145:214–222.

30. Reagan-Shaw S, Nihal M, Ahmad N. Dose translation from animal to human studies revisited. FASEB J. 2008; 22:659–661.

31. Kim NR, Lee JW, Jun SR, Lee IJ, Lim SD, Yeom JS, et al. Effects of epidural TNF-α inhibitor injection: analysis of the pathological changes in a rat model of chronic compression of the dorsal root ganglion. Skeletal Radiol. 2012; 41:539–545.

32. Sielenkämper AW, Eicker K, Van Aken H. Thoracic epidural anesthesia increases mucosal perfusion in ileum of rats. Anesthesiology. 2000; 93:844–851.

33. Dirks J, Møiniche S, Hilsted KL, Dahl JB. Mechanisms of postoperative pain: clinical indications for a contribution of central neuronal sensitization. Anesthesiology. 2002; 97:1591–1596.

34. Lavand'homme P. From preemptive to preventive analgesia: time to reconsider the role of perioperative peripheral nerve blocks? Reg Anesth Pain Med. 2011; 36:4–6.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download