Abstract
Purpose
Analyses of risk factors associated with surgical site infections (SSIs) after laparoscopic appendectomy (LA) have been limited. Especially, the association of an underweight body mass index (BMI) with SSIs has not been clearly defined. This study aimed to identify the impact of underweight BMI in predicting SSIs after LA.
Materials and Methods
The records of a total of 101 consecutive patients aged ≥16 years who underwent LA by a single surgeon between March 2011 and December 2012 were retrieved from a prospectively collected database. The rate of SSIs was compared among the underweight, normal and overweight and obese groups. Also, univariate and multivariate analyses were performed to identify the factors associated with SSIs.
Results
The overall rate of SSIs was 12.8%. The superficial incisional SSI rate was highest in the underweight group (44.4% in the underweight group, 11.0% in the normal group, and 0% in the overweight and obese group, p=0.006). In univariate analysis, open conversion and being underweight were determined to be risk factors for SSIs. Underweight BMI was also found to be a significant predictor for SSIs in multivariate analysis (odds ratio, 10.0; 95% confidence interval, 2.0-49.5; p=0.005).
Surgical site infections (SSIs) are defined as infections occurring within 30 days after a surgical operation, or within one year if an implant is left in place after the procedure, and affecting either the incision or deep tissue at the operation site.1 SSIs are the third most frequently reported nosocomial infections, accounting for 14-16% of such infections among hospitalized patients and 38% of such infections among surgical patients.1,2 SSIs are associated with a prolonged length of hospital stay and higher costs.3
Acute appendicitis is a common cause of emergency operations,4 for which laparoscopic appendectomy (LA) is being increasingly applied.5 LA provides several advantages, including less postoperative pain, early recovery, reduced morbidity, and shortened hospital stay in comparison to open appendectomy.6,7,8,9 Although it has been reported that LA reduces the rate of SSIs,7 the incidence of SSIs after LA ranged from 5.1-12.4% in recent well-designed randomized trials.10,11
To the best of our knowledge, analyses of the risk factors associated with SSIs after LA are limited. Additionally, although obesity is a well known risk factor for SSIs,12 the impact of being underweight as a risk factor of SSIs had been sparsely investigated.
Accordingly, the aim of this study was to identify the impact of underweight body mass index (BMI) in predicting SSIs after laparoscopic appendectomy.
Between March 2011 and December 2012, a total of 101 consecutive patients aged ≥16 years underwent laparoscopic appendectomy (LA) by a single surgeon. Patients that underwent open appendectomy performed during the same period were not included in this study. However, laparoscopic cases that were converted to an open procedure were not excluded from the intention-to-treat analysis. Patients' demographics, postoperative morbidity, pathologic outcomes, and parameters regarding immediate postoperative recovery were prospectively collected in our database. Informed consent was obtained from all patients before surgery.
Acute appendicitis was suspected on the basis of clinical signs (abdominal pain, rebound tenderness in the right lower quadrant) and laboratory test results [elevated white blood cell (WBC) count and serum C-reactive protein levels]. Diagnoses were confirmed by a contrast-enhanced abdominopelvic computed tomography scan or abdominal ultrasonography when needed.
All patients received second-generation cephalosporin intravenously at the induction of anesthesia. In preparation for the operation, the abdomen and suprapubic region were shaved. The umbilicus was cleaned thoroughly with a cotton swab using betadine. The abdomen was disinfected with betadine scrub (povidone-iodine) solution. A conventional three-port laparoscopic approach (using a 12-mm umbilical port with two additional 5-mm ports in the left lower abdomen and suprapubic area) was performed for all enrolled patients. The open method was routinely used for trocar insertion into the supraumbilical incision.
The mesoappendiceal tissue was dissected with monopolar electrocautery. The appendiceal artery was ligated with endo-clips. The appendix was ligated with three Endoloop ligatures and transected by endoscopic scissors between the 2nd and 3rd loop ligatures from the base of the cecum. The double-ligated appendiceal stump was cauterized and the transected appendix was retrieved from the peritoneal cavity using a Lap-bag through the supraumbilical incision. After transection and placing the appendix in the Lap-bag, irrigation and/or Jackson-Pratt drain insertion was performed in cases of suspected intraperitoneal contamination. The abdominal fascia layer at the supraumbilical incision was sutured using absorbable suture material. All three incisions were closed using skin staples. In the case of open conversion, a McBurney skin incision on the right lower abdomen or a paramedian skin incision were used for open appendectomy.
BMI was categorized according to the World Health Organization (WHO) definition: for adults over 20 years old, a BMI below 18.5 was underweight, 18.5-24.9 was normal weight, 25.0-29.9 was overweight, and above 30 was obese. In the case of 16-19 year olds, we used the BMI-for-age (5-19 years) cut-offs defined by the WHO: a BMI below -2 standard deviations (SD) was thin, between -2SD and +1SD was normal, over +1SD was overweight, and over +2SD was obesity.13,14 In this study, the thin, normal, overweight, and obesity groups used for 16-19 year olds were merged with the underweight, normal, overweight, and obese groups, respectively, in the analysis.
Postoperative complications were defined as any complications occurring in the 30 days after the day of surgery. All patients discharged from the hospital were followed up directly by the operator at the outpatient department. In addition, postoperative complications were also assessed by the operator during outpatient visit during the follow up period.
Complications were diagnosed and categorized by the patients' symptoms and signs. Superficial incisional SSI was defined according to the Centers for Disease Control and Prevention (CDC) criteria as follows: infection occurring within 30 days postoperatively and involving only skin and subcutaneous tissue of the incision. One of the following conditions must also be met: 1) purulent drainage from the superficial incision; 2) organisms isolated from an aseptic culture of fluid or tissue from the superficial incision; 3) at least one of the signs and symptoms such as pain or tenderness, localized swelling, redness or heat or superficial incision is deliberately opened by the surgeon irrespective of positive culture.15 Organ/Space SSI was also defined according to the CDC criteria.15
Ileus was defined as any condition with abdominal distension or pain with nausea or vomiting, which was confirmed with a plain X-ray. Postoperative outcomes, such as duration of soft diet resumed, and length of hospital stay were also analyzed. All perioperative outcomes were compared among three groups: underweight, normal, and overweight and obese group.
Categorical variables were analyzed using the chi-square or Fisher's exact test. All statistical tests were performed using SPSS version 20.0 (SPSS Inc., Chicago, IL, USA). The exact logistic regression analysis for the multivariate analysis of factors associated with SSIs was also performed. All p-values <0.05 were considered to indicate significance.
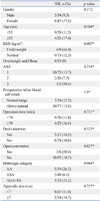
Patients were allocated into either the underweight group (n=9, 8.9%), normal group (n=73, 72.2%), or overweight and obese group (n=19, 18.8%). Patient demographics and perioperative surgical outcomes among the three groups are listed in Table 1. Notably, all patients in the underweight group were female (p=0.001). However, there were no differences in perioperative outcomes including age, American Society of Anesthesiology grade, history of previous abdominal surgery, mean of WBC count, operation time, open conversion rate, drain insertion rate, appendix size, histologic category, and recovery outcomes among the three groups.
Overall complication rate was highest in the underweight group (p=0.037). The rate of superficial incisional SSI was 44.4% in the underweight group, 16.4% in the normal group, and 0% in the overweight and obese group (p=0.006). There was no difference in the rate of organ/space SSI among the three groups.
Univariate analysis showed that open conversion and BMI were associated with SSIs (Table 2). These two factors were also found to be significant predictors for SSIs in multivariate analysis [open conversion-odds ratio (OR), 8.5; 95% confidence interval (CI), 1.2-57.8; p=0.027, BMI-others vs. underweight: OR, 10.0; 95% CI, 2.0-49.5; p=0.005] (Table 3).
The results of this study demonstrate that an underweight BMI is a risk factor for SSIs after laparoscopic appendectomy.
In patients with acute appendicitis, the incidence of SSIs has been reported to be 2.8-11.5% with laparoscopic appendectomy and 4.6-9.7% with open procedures.16,17,18,19 The incidence of SSIs in our study was 12.8%, which was higher than that in other reports. Retrospective studies with multiple surgeons might have had difficulties in including post discharge data precisely, which may lead to the underestimation of the incidence of SSIs. In our study, all patients were operated on and followed up in an outpatient department by a single surgeon (J. K.). Since the patients returned to the hospital voluntarily for their discomfort, even after cessation of follow-up, all SSIs could be detected and recorded precisely in the prospectively maintained database. Interestingly, the rate of SSIs in our study was very similar to that of the laparoscopic arm (12.4%) of a recent randomized-controlled trial conducted in Korea.10 In contrast, the rate of organ/space SSIs (0.9%) in our study was relatively lower than that in other reports.17,19,20 Although the reason for the low rate of organ/space SSIs is unclear, the operator always tried to remove any remaining dirty fluids in the pelvic cavity using position change: it has been reported that intra-abdominal pelvic abscesses can be reduced by complete irrigation and aspiration using Trendelenburg position.21
This study demonstrated that open conversion was a procedure-related risk factor for SSIs. Li, et al.22 reported a higher wound infection rate in the conversion group after laparoscopic-assisted right colon resection. However, the impact of open conversion on increased SSIs is controversial in LA. Piskun, et al.23 reported higher postoperative infectious morbidity in the converted group for perforated appendicitis. In contrast, Kouwenhoven, et al.24 reported a similar SSI rate between a conversion group and a laparoscopic group in patients with acute appendicitis. The close proximity to the abdominal wall during appendectomy could be a cause of wound infection.25 There may be a higher chance of contacting the abdominal wall in open conversion cases, compared to non-conversion cases, in LA. Our results reveal that open conversion is a risk factor for SSIs in LA. Therefore, surgeons should be more cautious in protecting abdominal incisions, especially in converted cases.
In our study, another risk factor for SSIs was an underweight BMI, but not overweight or obese BMI. This was an interesting result because a higher rate of SSIs among obese patients is a well-established finding.12 Due to the character of Asian populations, our study did not have enough patients with a BMI of 30 kg/m2 or more, which may have resulted in selection bias. However, being overweight (BMI 25.0-29.9) is also known to be a risk factor for SSIs in gastric or colorectal cancer surgery.26,27 Thus, it is difficult to attribute the correlation of an underweight BMI with SSIs due to the lack of obese patients in our cohort. Pessaux, et al.28 demonstrated that being underweight was one of the risk factors for global infectious complications in their study of 4718 patients of non-colorectal abdominal surgery. Being underweight was also significantly correlated with SSI, parietal complications, and deep infectious complications with or without fistulas in univariate analysis.28 This interrelationship between being underweight and the risk for infectious complications may be explained in terms of nutritional status. An underweight BMI may reflect malnutrition.29 It has been reported that malnutrition changes the defense system and its function against infection.30,31 Oh, et al.32 demonstrated that immediate postoperative malnutrition status as measured by the nutrition risk index was correlated with wound complications after gastrectomy. Therefore, an underweight BMI should be regarded as a modifiable factor to decrease infectious complications, as recommended by Pessaux, et al.28
In our study, SSI rate in acute appendicitis or acute non-specific appendicitis was marginally higher than that of other categories in univariate analysis (p=0.064). However, histologic grade did not show any difference of SSI rate in multivariate analysis. Because the final pathology was reported after the processing of histologic examinations, the pathology could not discriminate the difference of operative diagnosis in detail.19 Rather, we believe that clinical difficulties such as edematous appendix or gangrenous appendicitis with abscess formation were reflected in the parameter of open conversion in our analysis.
This study has several limitations, primarily related to the retrospective study design. We lacked data on certain serologic markers indicative of malnutrition status, such as serum albumin, prealbumin, and lymphocyte counts.33,34 These data were inconsistently documented and therefore not reliable for analysis. However, this limitation is balanced by the following strengths: standardized antibiotics use, LA conducted by a single surgeon, and the ability to gather detailed postoperative complications.
In conclusion, our data demonstrate that underweight is a risk factor that affects SSIs after laparoscopic appendectomy. In order to reduce SSIs, surgeons should be more cautious when operating on underweight patients.
Figures and Tables
References
1. Mangram AJ, Horan TC, Pearson ML, Silver LC, Jarvis WR. Centers for Disease Control and Prevention (CDC) Hospital Infection Control Practices Advisory Committee. Guideline for Prevention of Surgical Site Infection, 1999. Am J Infect Control. 1999; 27:97–132.

2. Emori TG, Gaynes RP. An overview of nosocomial infections, including the role of the microbiology laboratory. Clin Microbiol Rev. 1993; 6:428–442.

3. Urban JA. Cost analysis of surgical site infections. Surg Infect (Larchmt). 2006; 7:Suppl 1. S19–S22.

4. Addiss DG, Shaffer N, Fowler BS, Tauxe RV. The epidemiology of appendicitis and appendectomy in the United States. Am J Epidemiol. 1990; 132:910–925.

5. Saia M, Buja A, Baldovin T, Callegaro G, Sandonà P, Mantoan D, et al. Trend, variability, and outcome of open vs. laparoscopic appendectomy based on a large administrative database. Surg Endosc. 2012; 26:2353–2359.

6. Sauerland S, Jaschinski T, Neugebauer EA. Laparoscopic versus open surgery for suspected appendicitis. Cochrane Database Syst Rev. 2010; CD001546.

7. Wei HB, Huang JL, Zheng ZH, Wei B, Zheng F, Qiu WS, et al. Laparoscopic versus open appendectomy: a prospective randomized comparison. Surg Endosc. 2010; 24:266–269.

8. Tiwari MM, Reynoso JF, Tsang AW, Oleynikov D. Comparison of outcomes of laparoscopic and open appendectomy in management of uncomplicated and complicated appendicitis. Ann Surg. 2011; 254:927–932.

9. Kim CB, Kim MS, Hong JH, Lee HY, Yu SH. Is laparoscopic appendectomy useful for the treatment of acute appendicitis in Korea? A meta-analysis. Yonsei Med J. 2004; 45:7–16.

10. Lee WS, Choi ST, Lee JN, Kim KK, Park YH, Lee WK, et al. Single-port laparoscopic appendectomy versus conventional laparoscopic appendectomy: a prospective randomized controlled study. Ann Surg. 2013; 257:214–218.

11. Teoh AY, Chiu PW, Wong TC, Poon MC, Wong SK, Leong HT, et al. A double-blinded randomized controlled trial of laparoendoscopic single-site access versus conventional 3-port appendectomy. Ann Surg. 2012; 256:909–914.

12. Anaya DA, Dellinger EP. The obese surgical patient: a susceptible host for infection. Surg Infect (Larchmt). 2006; 7:473–480.

13. Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, Flegal KM, Guo SS, Wei R, et al. CDC growth charts: United States. Adv Data. 2000; 1–27.
14. Cole TJ, Bellizzi MC, Flegal KM, Dietz WH. Establishing a standard definition for child overweight and obesity worldwide: international survey. BMJ. 2000; 320:1240–1243.

15. Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control. 2008; 36:309–332.

16. Suh YJ, Jeong SY, Park KJ, Park JG, Kang SB, Kim DW, et al. Comparison of surgical-site infection between open and laparoscopic appendectomy. J Korean Surg Soc. 2012; 82:35–39.

17. Katkhouda N, Mason RJ, Towfigh S, Gevorgyan A, Essani R. Laparoscopic versus open appendectomy: a prospective randomized double-blind study. Ann Surg. 2005; 242:439–448.

18. Kehagias I, Karamanakos SN, Panagiotopoulos S, Panagopoulos K, Kalfarentzos F. Laparoscopic versus open appendectomy: which way to go? World J Gastroenterol. 2008; 14:4909–4914.

19. Pedersen AG, Petersen OB, Wara P, Rønning H, Qvist N, Laurberg S. Randomized clinical trial of laparoscopic versus open appendicectomy. Br J Surg. 2001; 88:200–205.
20. Wilson DG, Bond AK, Ladwa N, Sajid MS, Baig MK, Sains P. Intra-abdominal collections following laparoscopic versus open appendicectomy: an experience of 516 consecutive cases at a district general hospital. Surg Endosc. 2013; 27:2351–2356.

21. Katkhouda N, Friedlander MH, Grant SW, Achanta KK, Essani R, Paik P, et al. Intraabdominal abscess rate after laparoscopic appendectomy. Am J Surg. 2000; 180:456–459.

22. Li JC, Lee JF, Ng SS, Yiu RY, Hon SS, Leung WW, et al. Conversion in laparoscopic-assisted colectomy for right colon cancer: risk factors and clinical outcomes. Int J Colorectal Dis. 2010; 25:983–988.

23. Piskun G, Kozik D, Rajpal S, Shaftan G, Fogler R. Comparison of laparoscopic, open, and converted appendectomy for perforated appendicitis. Surg Endosc. 2001; 15:660–662.

24. Kouwenhoven EA, Repelaer van, van Erp WF. Fear for the intraabdominal abscess after laparoscopic appendectomy: not realistic. Surg Endosc. 2005; 19:923–926.

25. Lujan Mompean JA, Robles Campos R, Parrilla Paricio P, Soria Aledo V, Garcia Ayllon J. Laparoscopic versus open appendicectomy: a prospective assessment. Br J Surg. 1994; 81:133–135.
26. Hirao M, Tsujinaka T, Imamura H, Kurokawa Y, Inoue K, Kimura Y, et al. Overweight is a risk factor for surgical site infection following distal gastrectomy for gastric cancer. Gastric Cancer. 2013; 16:239–244.

27. Smith RL, Bohl JK, McElearney ST, Friel CM, Barclay MM, Sawyer RG, et al. Wound infection after elective colorectal resection. Ann Surg. 2004; 239:599–605.

28. Pessaux P, Msika S, Atalla D, Hay JM, Flamant Y. French Association for Surgical Research. Risk factors for postoperative infectious complications in noncolorectal abdominal surgery: a multivariate analysis based on a prospective multicenter study of 4718 patients. Arch Surg. 2003; 138:314–324.

29. Nishida T, Sakakibara H. Association between underweight and low lymphocyte count as an indicator of malnutrition in Japanese women. J Womens Health (Larchmt). 2010; 19:1377–1383.

31. Law DK, Dudrick SJ, Abdou NI. Immunocompetence of patients with protein-calorie malnutrition. The effects of nutritional repletion. Ann Intern Med. 1973; 79:545–550.

32. Oh CA, Kim DH, Oh SJ, Choi MG, Noh JH, Sohn TS, et al. Nutritional risk index as a predictor of postoperative wound complications after gastrectomy. World J Gastroenterol. 2012; 18:673–678.

33. Myron Johnson A, Merlini G, Sheldon J, Ichihara K. Scientific Division Committee on Plasma Proteins (C-PP), International Federation of Clinical Chemistry and Laboratory Medicine (IFCC). Clinical indications for plasma protein assays: transthyretin (prealbumin) in inflammation and malnutrition. Clin Chem Lab Med. 2007; 45:419–426.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download