Abstract
Purpose
Teriparatide markedly increases bone formation and strength, while reducing the incidence of new-onset osteoporotic vertebral compression fractures (OVCFs). In some countries, expenses for teriparatide use are covered by medical insurance for up to 6 months; however, the national medical insurance of the authors' country does not cover these expenses. This retrospective cohort study compared the therapeutic effects of teriparatide on the initial onset of a new OVCF after treatment of osteoporosis and/or related OVCFs with regard to therapeutic durations of longer than 3 months (LT3M) or shorter than 3 months (ST3M).
Materials and Methods
From May 2007 to February 2012, 404 patients who were prescribed and administered teriparatide and who could be followed-up for longer than 12 months were enrolled. They were divided into two groups depending on teriparatide duration: LT3M (n=132) and ST3M (n=272).
Results
The group with the teriparatide duration of LT3M showed significantly less development of an initial OVCF within 1 year (p=0.004, chi-square). Duration of teriparatide use, body mass index, pre-teriparatide lowest spinal bone mineral density, and severity of osteoporosis significantly affected multiple regression analysis results (p<0.05). Survival analysis of first new-onset OVCFs demonstrated a significantly better survival rate for the LT3M group (log rank, p=0.005). Also, the ST3M group showed a higher odds ratio of 54.00 for development of an initial OVCF during follow-up than the LT3M group (Mantel-Haenzel common odds ratio, p=0.006).
Osteoporotic vertebral compression fractures (OVCFs) are the most common fragility fracture and are a hallmark of osteoporosis. OVCFs are associated with significantly decreased quality of life and increased mortality in the elderly.1,2 Nowadays, multi-disciplinary approaches to prevent OVCFs, including prevention of falls and administration of osteoporosis medications, are mandatory.3,4 Among different osteoporosis medications, teriparatide was the first anabolic agent approved by the U.S Food and Drug Administration to treat osteoporosis.5 Teriparatide involves a direct mechanism that stimulates bone formation and improves bone strength faster than other anti-resorptive osteoporosis medications.4,6,7,8,9
The dramatic bone formation seen with teriparatide therapy peaks at 6 to 12 months of therapy.10 However, the high price of teriparatide therapy (800 US dollars/month), roughly 30-fold higher than weekly bisphosphonate for a month, sometimes hampers its continuous use in clinical settings. Moreover, studies have yet to suggest clinical guidelines for preventing new OVCFs in patients using teriparatide, in particular how long teriparatide should be administered to ensure protective effects against the development of new-onset OVCFs.
In the present study, we investigated the minimum period of teriparatide use necessary to ensure prevention of additional new-onset OVCFs. Additional correlation analyses were also conducted to determine the initial onset of a new OVCF after teriparatide treatment in a clinical setting according to duration of teriparatide administration.
This study was approved by the Institutional Review Board of the authors' hospital (IRB No. 3-2012-0221).
From May 2007 to February 2012, 650 patients were prescribed and administered teriparatide for at least 1 month for treatment of osteoporosis and/or OVCFs as suggested by guidelines for osteoporosis treatment.3,11 Among these, 430 patients who were followed-up for longer than 12 months were enrolled in the present study.
Additionally, data from patients who met the following exclusion criteria were removed from analysis: 1) musculoskeletal conditions that altered bone condition, such as arthrogryposis multiplex congenita, neuromuscular scoliosis, and Down syndrome; 2) patients who had a new onset OVCF from major trauma, such as a fall from a height or traffic accidents during follow-up; and 3) patients in whom complications related to teriparatide use were detected, such as generalized weakness and hypercalcemia.
A total of 26 patients were excluded from the patient pool, and 404 patients were finally enrolled. Basic data related to teriparatide administration [20 mcg/day, 250 mcg/mL×2.4 mL for 1 pen (28 days duration), Lilly Korea] were collected from electronic medical records.
In all enrolled patients, dual energy X-ray absorptiometry was performed using a GE-lunar densitometer (Lunar Prodigy, GE Lunar Corp., Madison, WI, USA). This technique was used to measure areal bone mineral density (BMD) of the lumbar spine previous to teriparatide administration, and measurements were repeated annually. An established visual semi-quantitative system was used to evaluate the presence of OVCFs12 on conventional thoraco-lumbar lateral radiographs taken at regular follow-up visits on a monthly basis for up to 3 months after first prescription of teriparatide. Then, patients were allowed to visit freely when they consumed all the osteoporosis medication or newly developed back pain was detected. To confirm a new onset OVCF, magnetic resonance imaging was performed in patients with new onset back pain and/or a new suspected OVCF on follow-up lateral plain radiographs during follow-up.
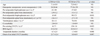
Enrolled patients were divided into two groups based on teriparatide duration: shorter than 3 months (ST3M) or longer than 3 months (LT3M). Basic data were compared between the two groups (Table 1). Co-existing degenerative spine conditions consisted of spinal stenosis (118 patients), spinal stenosis with spondylolisthesis (27 patients), degenerative scoliosis (29 patients), and herniated nucleus pulposus (25 patients).
An independent t-test was used to compare the results between groups. Chi-square test was conducted to analyze the distribution of initial OVCFs depending on duration of teriparatide use. Mantel-Haenzel common odds ratio analysis was used to estimate the odds ratio of the development of a new OVCF between the two groups. Forward stepwise multiple regression analyses were used to investigate factors affecting the development of the initial OVCFs, including age, gender, severity of osteoporosis, duration of teriparatide administration, previous history of vertebroplasty (VP), lowest femoral BMD score before teriparatide administration, lowest spine BMD score before teriparatide administration, presence of pre-existing OVCFs, body mass index (BMI), types and duration of pre-teriparatide and post-teriparatide osteoporosis medications, and co-morbidities. Kaplan-Meier survival analysis and a log-rank test were used to compare initial OVCF development between the ST3M and LT3M groups. Additional statistical analyses including chi-square and survival analyses were also performed to investigate the effect of VP status and preexisting OVCFs status on the development of an initial OVCF. All statistical analyses were performed using the SPSS 12.0.1 statistics package (SPSS Inc., Chicago, IL, USA). p-values <0.05 were considered statistically significant.
The mean age of enrolled patients was 72.5 years (50-91 years). Four hundred and four postmenopausal females were enrolled. The mean follow-up period was 27.0±13.9 months (12-57 months).
Before teriparatide use, the lowest spine BMD was -3.1±1.2 and the lowest femoral BMD, other than the femoral Ward's triangle, was -2.5±1.0. The mean duration teriparatide was 3.3±2.8 months. Mean BMI was 22.5±3.5. The mean number of co-morbidities was 1.9±0.9. Mean onset of an initial OVCF was 13.3±12.8 months after the first teriparatide administration.
The mean results of pre-teriparatide serologic tests were 93.9±29.1 ng/dL of T3 (normal range 71-161), 6.21±3.54 mcg/dL of T4 (normal range 5.5-10.6), 0.52±0.34 ng/mL of C-terminal telopeptide (CTX) (normal range 0.11-1.00), 27.4±27.2 ng/mL of osteocalcin (OC) (normal range 8.0-44.0), 44.5±25.2 pg/mL of intact parathyroid hormone (PTH) (normal range 13-104), 56.9±36.0 nM BCE/nM Cr of N-terminal telopeptide (NTX) (normal range 6.0-125.72), 8.7±0.6 mg/dL of calcium (normal range 8.6-10.0), 3.6±0.9 mg/dL of inorganic phosphate (normal range 2.8-4.5), and 34.15±19.0 ng/mL of 25-OH vitamin D3.
The 1-year post-teriparatide follow-up lab analysis revealed 76.8±22.3 ng/dL of T3, 4.6±3.7 mcg/dL of T4, 0.54±0.55 ng/mL of CTX, 43.1±22.7 ng/mL of OC, 25.8±14.1 pg/mL of PTH, 78.0±34.1 nM BCE/nM Cr of NTX, 9.0±1.4 mg/dL of calcium, 3.6±0.5 mg/dL of inorganic phosphate, and 36.7±11.2 ng/mL of 25-OH vitamin D.
From a total of 404 patients, 272 (67.3%) patients were categorized in the ST3M group and 132 (32.7%) patients in LT3M group. Table 1 shows a comparison between ST3M and LT3M groups. Among many factors, only the duration of teriparatide use was significantly different between the two groups (p=0.000, independent t-test).
A total of 17 (4.2%) patients exhibited an initial OVCF during follow-up. There was no difference in the overall development of an initial OVCF between ST3M (10 cases among 272 cases, 3.7%) and LT3M (seven cases among 132 cases, 5.3%) groups. However, there was a significantly different distribution of initial OVCFs within 1 year, which developed less in the LT3M group (one of seven first new OVCFs) than the ST3M group (nine of 10 first new OVCFs) (p=0.004, chi-square) (Fig. 1).
Prior to teriparatide administration, only 102 patients (25.2%) had been treated for osteoporosis and were taking calcium-vitamin D supplements (31 patients) or bisphosphonate (71 patients). The mean duration of taking calcium-vitamin D supplements was 3.7±6.5 months. The mean duration of bisphosphonate medication was 11.9±17.6 months.
After teriparatide administration, 249 patients (61.6%) taking calcium-vitamin D supplements (87 patients) or bisphosphonate (162 patients) were treated for osteoporosis. The mean duration of taking calcium-vitamin D supplements during follow-up was 4.0±4.4 months. The mean duration of bisphosphonate medication after teriparatide administration was 11.7±9.8 months. There was no difference in type and duration of pre-teriparatide osteoporosis medication between initial OVCF (+) and initial OVCF (-) patients during follow-up.
Two of (1.7%) 115 cases of osteopenia, two of (3.1%) 64 cases of osteoporosis, and 14 (6.2%) of 225 cases of severe osteoporosis (<-3.0) were confirmed to have an initial OVCF during follow-up. There was no statistical difference in the development of an initial OVCF between osteopenia, osteoporosis, and severe osteoporosis groups.
Among 404 patients, 217 (53.7%) patients had preexisting OVCFs and 183 (45.3%) patients did not. Six cases of an initial OVCF were detected among preexisting OVCFs (-) patients and 11 cases of an initial OVCF were detected in preexisting OVCFs (+) patients. There was no statistical difference according to preexisting OVCFs status.
Ninety-six (23.8%) patients underwent VP before teriparatide administration. Depending on VP status, a significant difference in the development of an initial OVCF was detected. Nine (2.9%) of 314 non-VP patients and eight (8.9%) of 90 VP patients showed an initial OVCF (p=0.027, chi-square). Among VP patients, adjacent OVCFs were observed in four (50%) cases, while all others were distant OVCFs.
Survival analysis of a first new onset OVCF showed a statistical difference in the survival rates of both groups (log rank, p=0.005) (Fig. 2).
In the LT3M group, only one of 7 patients developed an initial OVCF within 12 months. In the ST3M group, most (nine of 10) initial OVCFs occurred within 1 year (Fig. 2).
There was a statistical difference between the survival rates depending on preexisting OVCFs (+) and (-) status (log rank, p=0.005) (Fig. 3). The preexisting OVCF (+) group showed an even distribution of initial OVCFs (11 cases) during follow-up. The preexisting OVCF (-) group recorded one out of six cases at 3 month follow-up and other five cases after 24 months of follow-up.
The VP (+) group and VP (-) group showed statistically different survival rates (log rank, p=0.000) (Fig. 4). All new OVCFs in 8 cases in the previous VP (+) group occurred within 1 year during follow-up. The previous VP (-) group showed an even distribution of nine initial OVCFs during 24 months of follow-up.
In multiple regression analysis, test results were significantly affected by duration of teriparatide use, BMI, lowest pre-teriparatide spine BMD, and severity of osteoporosis (p<0.05) (Table 2). Age, pre-existing OVCFs, VP status, lowest pre-teriparatide femoral BMD, types and duration of pre-teriparatide and post-teriparatide osteoporosis medication, pre- and post-teriparatide bisphosphonate medication and co-morbidity did not affect the development of a first new OVCF.
Also, the ST3M group showed higher odds ratio of 54.000 (95% confidence interval: 2.804-1040.048) for development of an initial OVCF during follow-up than the LT3M group (Mantel-Haenzel common odds ratio estimates, p=0.006).
Subcutaneous teriparatide injection was previously shown to be associated with increased trabecular bone, cortical parameters, bone formation rates, and successive fracture prevention to reduce fracture risk.5,8,13,14 In a published meta-analysis of clinical trials, patients treated with teriparatide exhibited a reduced incidence of back pain and also an increased quality of life relative to patients receiving a placebo and anti-resorptive drugs.15,16,17
One major concern with teriparatide is its high price. Because teriparatide costs more than 800 US dollars per month in the authors' country, it is not uncommon to meet patients who insist on changing to other osteoporosis medications, such as bisphosphonate, which costs roughly 30 US dollars per month. In some countries, expenses for teriparatide use are covered by medical insurance for up to 6 months; however, the national medical insurance of the authors' country does not cover these expenses. To reflect this clinical background, in the present study, only 32.7% of enrolled patients were administered teriparatide for longer than 3 months.
Different from the clinical setting of the present study, many clinical results have been determined based on long-term teriparatide use for 12 to 24 months.8,10 Even short-term treatment with teriparatide was proven effective at increasing cancellous, endocortical, and periosteal bone formation rates.18 In a teriparatide fracture prevention trial, teriparatide reduced the risk of new vertebral fractures and nonvertebral fractures, but unfortunately, the study was stopped early because of the discovery of increased risk of osteosarcoma in animal models.5,8
One purpose of the present study was to investigate how long physicians should recommend candidates for such an expensive and possibly osteosarcoma-related medication to prevent new OVCFs. As expected, 3 months of teriparatide use, which the authors considered compatible with the normal physiologic bone cycle,19,20 showed a different pattern of initial OVCF development. Nevertheless, the authors failed to show a reduction in the total incidence of initial OVCFs for the LT3M group, compared to the ST3M group. However, the LT3M group demonstrated at least the delaying of or protective effects of teriparatide on the development of initial OVCFs in terms of recovering and strengthening bone quality in osteoporotic patients (Fig. 1). Paying closer attention to the interpretation of Fig. 1, the authors did not attempt to show an increase in the development of new OVCFs in the LT3M group after 1 year, but rather wanted to demonstrate the delaying effect of teriparatide on the development of an initial OVCF after treatment between the two groups. The mean new OVCFs free periods were four times longer in the LT3M group than in the ST3M group (Table 1). Finally, the longer period free from a new OVCF in the LT3M group would translate to greater cost-effectiveness in clinical settings, reducing OVCF-related social-economic burdens stemming from the use of teriparatide, compared with other osteoporosis medications.21,22 Additionally, we confirmed a higher odds ratio for the development of an initial OVCF within 1 year in the ST3M group than the LT3M group. Therefore, the present authors highly encourage the use of teriparatide for longer than 3 months.
As for compliance of teriparatide administration, interestingly, because teriparatide is expensive, most of the patients that underwent daily teriparatide self-injection did not want to miss a single dose for 1 month. Also, post-teriparatide osteoporosis treatment ratio increased.
In the present study, pre-teriparatide osteoporosis medication use was a major confounding factor, which could have affected the development of initial OVCFs. Unfortunately for patients, the administration of osteoporosis medications prior to teriparatide therapy was very low (25.2% only), and the ratio of taking bisphosphonate, which is a stronger confounding factor than calcium-vitamin D supplements, was only 17% among enrolled patients. Therefore, the authors could easily exclude confounding effects related to previous bisphosphonate medication use.
The present study also confirmed the possible detrimental effects of presence of preexisting OVCFs and VP status during teriparatide use. Sagittal imbalance of the whole spine caused by preexisting OVCFs could contribute to the different distributions of development of an initial OVCF due to biomechanical uneven axial loading on adjacent vertebrae (Fig. 3), as a fully restored augmented vertebral body with appropriate sagittal alignment would be the goal of treatment.21,22 Although teriparatide improves bone strength faster than other anti-resorptive agents,7,8,23,24 all first new OVCFs developed within 1 year in VP patients (Fig. 4). Patients who undergo VP have been reported to have a greater risk of new-onset OVCFs in adjacent and non-adjacent spinal levels, compared to patients with prior OVCFs who did not undergo VP,25,26 which supports recent studies that have questioned the effects of VP.7,26,27,28,29,30,31 Therefore, VP status and presence of preexisting OVCFs also must be closely monitored in patients administered teriparatide to prevent new onset OVCFs.
In addition, multiple regression analysis in the present study also showed that BMI, severity of osteoporosis, and low BMD affect the development of initial OVCFs with teriparatide use correspondent to previous studies.32,33,34 Also, considering pre- and posterior teriparatide as factors affecting the development of initial OVCFs, the authors showed that short term use of bisphosphonate and calcium-vitamin D supplements did not affect the development of initial OVCFs. Therefore, this would support a stronger effect for teriparatide, compared to a long duration of bisphosphonate use, even with a mean duration of 6.7 months in the LT3M group, with 11.9 months of pre-teriparatide and 11.7 months of post-teriparatide use.
Limitations of the present study include lack of a pain scale during follow-up due to the retrospective study design and a failure to control possible confounding factors, such as pre-teriparatide and/or post-teriparatide osteoporosis medication. Nevertheless, the major finding of this study suggests that at least 3 months of teriparatide use should be the new standard in patients with high fracture risk or intolerance to other osteoporosis therapies.35 Also, the present authors focused on the development of an initial OVCF instead of development of all OVCFs during follow-up.
In conclusion, at least 3 months of cyclic teriparatide use was associated with lower risk and delayed onset time for an initial OVCF after treatment during follow-up. At least 3 months of continuous teriparatide administration is suggested for treating osteoporosis and related OVCFs. This provides a protective effect against development of an initial OVCF for up to one year after therapy.
Figures and Tables
Fig. 1
Distribution of an initial OVCF depending on teriparatide duration. The group with teriparatide duration of LT3M exhibited a statistically different distribution pattern of initial OVCFs within 1 year, compared to that of the group with teriparatide duration of ST3M (p=0.004, chi-square). The mean onset time of an initial OVCF was 23.7±12.4 months in the LT3M group and 6.0±6.9 months in the ST3M group (Mann-Whitney U test, p=0.007). Also, the ST3M group showed higher odds ratio of 54.000 (95% confidence interval: 2.804-1040.048) for the development of an initial OVCF during follow-up than the LT3M group (Mantel-Haenzel common odds ratio estimates, p=0.006). OVCFs, osteoporotic vertebral compression fractures; LT3M, longer than 3 months; ST3M, shorter than 3 months.

Fig. 2
Survival analysis of an initial OVCF depending on teriparatide duration. We observed a statistically significant difference in the occurrence of an initial OVCF between groups, which was better in the teriparatide duration of LT3M group (log rank, p=0.005). In the LT3M group, only one of 7 patients developed an initial OVCF within 12 months. In the ST3M group, most (9 of 10) initial OVCFs occurred within 1 year. OVCFs, osteoporotic vertebral compression fractures; LT3M, longer than 3 months; ST3M, shorter than 3 months.

Fig. 3
Survival analysis of an initial OVCF depending on the presence of preexisting OVCFs. The occurrence of an initial OVCF in the preexisting OVCF (+) group was statistically different from those of the preexisting OVCF (-) group (log rank, p=0.005). The preexisting OVCF (+) group showed an even distribution of initial OVCFs (11 cases) during follow-up. The preexisting OVCF (-) group recorded one case out of six cases at 3 month follow-up and another five cases after 24 months of follow-up. OVCFs, osteoporotic vertebral compression fractures.

Fig. 4
Survival analysis of an initial OVCF depending on VP status. There was a significantly different survival rate for the previous VP (+) group and VP (-) group (log rank, p=0.000). All initial OVCFs in eight cases in the previous VP (+) group occurred within 1 year during follow-up. The previous VP (-) group showed an even distribution of nine initial OVCFs during 24 months of follow-up. OVCFs, osteoporotic vertebral compression fractures; VP, vertebroplasty.

References
2. Hall SE, Criddle RA, Comito TL, Prince RL. A case-control study of quality of life and functional impairment in women with long-standing vertebral osteoporotic fracture. Osteoporos Int. 1999; 9:508–515.

3. Kanis JA, Black D, Cooper C, Dargent P, Dawson-Hughes B, De Laet C, et al. A new approach to the development of assessment guidelines for osteoporosis. Osteoporos Int. 2002; 13:527–536.

4. Ettinger B, Black DM, Mitlak BH, Knickerbocker RK, Nickelsen T, Genant HK, et al. Multiple Outcomes of Raloxifene Evaluation (MORE) Investigators. Reduction of vertebral fracture risk in postmenopausal women with osteoporosis treated with raloxifene: results from a 3-year randomized clinical trial. JAMA. 1999; 282:637–645.

5. Uihlein AV, Leder BZ. Anabolic therapies for osteoporosis. Endocrinol Metab Clin North Am. 2012; 41:507–525.

6. Black DM, Cummings SR, Karpf DB, Cauley JA, Thompson DE, Nevitt MC, et al. Fracture Intervention Trial Research Group. Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Lancet. 1996; 348:1535–1541.

7. Tseng YY, Su CH, Lui TN, Yeh YS, Yeh SH. Prospective comparison of the therapeutic effect of teriparatide with that of combined vertebroplasty with antiresorptive agents for the treatment of new-onset adjacent vertebral compression fracture after percutaneous vertebroplasty. Osteoporos Int. 2012; 23:1613–1622.

8. Neer RM, Arnaud CD, Zanchetta JR, Prince R, Gaich GA, Reginster JY, et al. Effect of parathyroid hormone (1-34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med. 2001; 344:1434–1441.

9. Park JH, Kang KC, Shin DE, Koh YG, Son JS, Kim BH. Preventive effects of conservative treatment with short-term teriparatide on the progression of vertebral body collapse after osteoporotic vertebral compression fracture. Osteoporos Int. 2014; 25:613–618.

10. Finkelstein JS, Wyland JJ, Lee H, Neer RM. Effects of teriparatide, alendronate, or both in women with postmenopausal osteoporosis. J Clin Endocrinol Metab. 2010; 95:1838–1845.

11. Kanis JA, McCloskey EV, Johansson H, Oden A, Ström O, Borgström F. Development and use of FRAX in osteoporosis. Osteoporos Int. 2010; 21:Suppl 2. S407–S413.

12. Binkley N, Krueger D, Gangnon R, Genant HK, Drezner MK. Lateral vertebral assessment: a valuable technique to detect clinically significant vertebral fractures. Osteoporos Int. 2005; 16:1513–1518.

13. Jiang Y, Zhao JJ, Mitlak BH, Wang O, Genant HK, Eriksen EF. Recombinant human parathyroid hormone (1-34) [teriparatide] improves both cortical and cancellous bone structure. J Bone Miner Res. 2003; 18:1932–1941.

14. Dempster DW, Cosman F, Kurland ES, Zhou H, Nieves J, Woelfert L, et al. Effects of daily treatment with parathyroid hormone on bone microarchitecture and turnover in patients with osteoporosis: a paired biopsy study. J Bone Miner Res. 2001; 16:1846–1853.

15. Nevitt MC, Chen P, Dore RK, Reginster JY, Kiel DP, Zanchetta JR, et al. Reduced risk of back pain following teriparatide treatment: a meta-analysis. Osteoporos Int. 2006; 17:273–280.

16. Nevitt MC, Chen P, Kiel DP, Reginster JY, Dore RK, Zanchetta JR, et al. Reduction in the risk of developing back pain persists at least 30 months after discontinuation of teriparatide treatment: a meta-analysis. Osteoporos Int. 2006; 17:1630–1637.

17. Ulivieri FM. Back pain treatment in post-menopausal osteoporosis with vertebral fractures. Aging Clin Exp Res. 2007; 19:3 Suppl. 21–23.
18. Lindsay R, Zhou H, Cosman F, Nieves J, Dempster DW, Hodsman AB. Effects of a one-month treatment with PTH(1-34) on bone formation on cancellous, endocortical, and periosteal surfaces of the human ilium. J Bone Miner Res. 2007; 22:495–502.

19. Martin TJ, Sims NA, Ng KW. Regulatory pathways revealing new approaches to the development of anabolic drugs for osteoporosis. Osteoporos Int. 2008; 19:1125–1138.

20. Cosman F, Nieves J, Zion M, Woelfert L, Luckey M, Lindsay R. Daily and cyclic parathyroid hormone in women receiving alendronate. N Engl J Med. 2005; 353:566–575.

21. Nshimyumukiza L, Durand A, Gagnon M, Douville X, Morin S, Lindsay C, et al. An economic evaluation: simulation of the cost-effectiveness and cost-utility of universal prevention strategies against osteoporosis-related fractures. J Bone Miner Res. 2013; 28:383–394.

22. Wasserfallen JB, Krieg MA, Greiner RA, Lamy O. Cost effectiveness and cost utility of risedronate for osteoporosis treatment and fracture prevention in women: a Swiss perspective. J Med Econ. 2008; 11:499–523.

23. McClung MR, San Martin J, Miller PD, Civitelli R, Bandeira F, Omizo M, et al. Opposite bone remodeling effects of teriparatide and alendronate in increasing bone mass. Arch Intern Med. 2005; 165:1762–1768.

24. Body JJ, Gaich GA, Scheele WH, Kulkarni PM, Miller PD, Peretz A, et al. A randomized double-blind trial to compare the efficacy of teriparatide [recombinant human parathyroid hormone (1-34)] with alendronate in postmenopausal women with osteoporosis. J Clin Endocrinol Metab. 2002; 87:4528–4535.

25. Mudano AS, Bian J, Cope JU, Curtis JR, Gross TP, Allison JJ, et al. Vertebroplasty and kyphoplasty are associated with an increased risk of secondary vertebral compression fractures: a population-based cohort study. Osteoporos Int. 2009; 20:819–826.

26. Boger A, Heini P, Windolf M, Schneider E. Adjacent vertebral failure after vertebroplasty: a biomechanical study of low-modulus PMMA cement. Eur Spine J. 2007; 16:2118–2125.

27. Buchbinder R, Kallmes DF. Vertebroplasty: when randomized placebo-controlled trial results clash with common belief. Spine J. 2010; 10:241–243.

28. Buchbinder R, Osborne RH, Ebeling PR, Wark JD, Mitchell P, Wriedt C, et al. A randomized trial of vertebroplasty for painful osteoporotic vertebral fractures. N Engl J Med. 2009; 361:557–568.

29. Kallmes DF, Comstock BA, Heagerty PJ, Turner JA, Wilson DJ, Diamond TH, et al. A randomized trial of vertebroplasty for osteoporotic spinal fractures. N Engl J Med. 2009; 361:569–579.

30. Trout AT, Kallmes DF, Kaufmann TJ. New fractures after vertebroplasty: adjacent fractures occur significantly sooner. AJNR Am J Neuroradiol. 2006; 27:217–223.
31. Voormolen MH, Lohle PN, Lampmann LE, van den Wildenberg W, Juttmann JR, Diekerhof CH, et al. Prospective clinical follow-up after percutaneous vertebroplasty in patients with painful osteoporotic vertebral compression fractures. J Vasc Interv Radiol. 2006; 17:1313–1320.

32. Ravn P, Cizza G, Bjarnason NH, Thompson D, Daley M, Wasnich RD, et al. Early Postmenopausal Intervention Cohort (EPIC) study group. Low body mass index is an important risk factor for low bone mass and increased bone loss in early postmenopausal women. J Bone Miner Res. 1999; 14:1622–1627.

33. Moon ES, Kim HS, Park JO, Moon SH, Lee HM, Shin DE, et al. The incidence of new vertebral compression fractures in women after kyphoplasty and factors involved. Yonsei Med J. 2007; 48:645–652.

34. Zhang Z, Fan J, Ding Q, Wu M, Yin G. Risk factors for new osteoporotic vertebral compression fractures after vertebroplasty: a systematic review and meta-analysis. J Spinal Disord Tech. 2013; 26:E150–E157.
35. Blick SK, Dhillon S, Keam SJ. Teriparatide: a review of its use in osteoporosis. Drugs. 2008; 68:2709–2737.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download