Abstract
Purpose
Pouch dilatation and band slippage are the most common long-term complications after laparoscopic adjustable gastric banding (LAGB). The aim of the study is to present our experience of diagnosis and management of these complications.
Materials and Methods
The pars flaccida technique with anterior fixation of the fundus was routinely used. All band adjustments were performed under fluoroscopy. We analyzed the incidence, clinico-radiologic features, management, and revisional surgeries for treatment of these complications. We further presented the outcome of gastric plication techniques as a measure for prevention of these complications.
Results
From March 2009 to March 2012, we performed LAGB on 126 morbidly obese patients. Among them, 14 patients (11.1%) were diagnosed as having these complications. Four patients (3.2%) had concentric pouch dilatations, which were corrected by band adjustment. Ten (7.9%) had eccentric pouch with band slippage. Among the ten patients, there were three cases of posterior slippage, which were corrected by reoperation, and seven cases of eccentric pouch dilatation with anterior slippage. Three were early anterior slippage, which was managed conservatively. Two were acute anterior slippage, one of whom underwent a revision. There were two cases of chronic anterior slippage, one of whom underwent a revision. The 27 patients who underwent gastric plication did not present with eccentric pouch with band slippage during the follow-up period.
Obesity has reached epidemic proportions worldwide. Obesity is often associated with metabolic disorders such as type 2 diabetes, hypertension, and dyslipidemia, and subsequently causes an increased cardiovascular disease risk. Bariatric surgery is known to be the most effective and long lasting treatment for obesity and its comorbidities. Due to ease of performance, safety, and adjustability, laparoscopic adjustable gastric band (LAGB) surgery is one of the most popular bariatric procedures worldwide. Unlike gastric bypass and sleeve gastrectomy, it does not alter the anatomy of the GI tract. However, as the number of patients undergoing LAGB increases, reports on long-term complications, including pouch dilatation, band slippage, band erosion, and port-tube problems, have been considerable.1-4 Pouch dilatation and band slippage are troublesome problems for both patients and clinicians. Usual symptoms include reflux, vomiting, chest/abdominal pain, and partial or total food intolerance, in addition to suboptimal weight loss, resulting from intolerance to proper band adjustment. Although rare, these complications can be life-threatening.5,6 Therefore, careful clinico-radiologic follow-up of patients fitted with gastric bands, with a particular focus on these complications, is mandatory. This report reviews the outcome of 126 patients who underwent LAGB performed by a single surgeon during a period of three years for management of pouch dilatation with/without band slippage. Other complications of LAGB in our patient cohort, including band erosion, mechanical complication of the port, and tubing, have been previously described,7,8 and, for simplicity, were not included in the analysis. Based on the outcomes of the study, we attempted to answer the four following questions: 1) what is the incidence of these complications, especially at the beginning of the practice? 2) What are the most probable causes of these complications? 3) How are these complications managed individually? 4) Are there any ideal operative techniques for revision that can be used in correction of these complications? In addition, we compared outcomes of the gastric plication technique, which was performed primarily during the later period among our cohort of patients.
We followed guidelines from the Asian Consensus Meeting on Metabolic Surgery (2008, Trivandrum, India) for BMI restriction using bariatric surgery (http://www.acmoms.com/acmom_2008.html). We followed the operative techniques used in previous studies reported by large-scale bariatric centers.9,10 All operations were performed by the same surgeon utilizing LAP-BAND® (Allergan, Irvine, CA, USA) with different modifications (9.75/10.0, AP series). First, a very small pouch was formed by placement of the band on top of the stomach just below the gastroesophageal junction. A calibration tube with a 25 cc balloon was used for proper band placement and detection of the hiatal defect. Second, the pars flaccida technique was performed, in which entry into the lesser sac was avoided by placement of the band suprabursally around gastric vessels and fat instead of close to the gastric wall. Finally, anterior fixation of the gastric fundus was performed using three to four nonabsorbable sutures between above and below the band. In addition, for patients who underwent LAGB during the later period of the study, we performed routine oblique plication of the anterior gastric wall below the band after noting that the angle of the band appeared to be stabilized in patients despite the variable degree of food trouble (Fig. 1). Intraoperative band adjustment was not performed, and bands were left unfilled at the end of the procedure.
The protocol for band adjustment was as follows. The band was left empty at completion of band placement. The first fill was performed around four weeks postopertaively, at which time the postoperative edema subsided and patients began to regain initial small changes in weight. Subsequent band adjustments were performed at intervals of at least two weeks. Three initial adjustments were performed under fluoroscopy. Based on movement of the barium swallow, the diameter of the stoma was adjusted to 4 mm (identical to the thickness of the LAP-BAND® tubing). Thereafter, depending on the amount of change in body weight, portion size of food, and hunger, small volume adjustments were performed without a barium swallow (usually, 0.1-0.3 cc saline was added or removed). For particular symptoms, such as vomiting, reflux, and abdominal pain, we also performed a barium swallow study during the adjustment period. Pouch dilatation was classified as concentric and eccentric. Eccentric pouch dilatation was always accompanied by band slippage, which was classified as anterior and postoperative slippage. Therefore, complications of pouch dilatation with/without band slippage were codified as follows: CP for concentric pouch dilatation (normal band angle) (Fig. 2), EPA1 for eccentric pouch with early anterior slippage (normal band angle with a ring-like configuration), EPA2 for eccentric pouch with chronic anterior slippage (a more horizontal band angle), EPA3 for eccentric pouch with acute anterior slippage (an overwhelming pouch covers the band or clockwise rotation of the band with downward migration), and EPP for eccentric pouch with posterior slippage (the band was noted as having counterclockwise rotation with a huge air-fluid pocket inferior and posterior of the band) (Fig. 3). Conservative treatment included total or near-total removal of saline in the band and gradual readjustment with great care for any recurrent abnormal clinico-radiologic signs and symptoms. Strict dietary education was also administered during the 'rest' period. Readjustment was usually started around three to four weeks after total removal of saline. Patients who did not respond to the initial conservative management underwent revision. This revision always involved laparoscopic non-destructive removal of the band and its repositioning at a proper level; however, a more proximal retrogastric tunnel and more proximal to the enlarged proximal pouch, as described previously.11,12 During performance of revision surgery, we also performed oblique (anterolateral) and vertical (posterolateral) plications, which was fixation of the top of the neofundus onto the left pillar of the diaphragm crura (Fig. 4). During postoperative management, as with the original operation, patients underwent a gastrograffin esophagogram the morning after surgery and were discharged after demonstrating tolerance on a liquid diet.
During the study period, a total of 14 patients out of 126 patients (11.1%) were diagnosed as having pouch dilatation with/without band slippage. Thirteen of these patients were female. The mean±SD of the 14 patients for age and BMI measured at their first banding surgery was 31.9±8.1 years and 36.1±4.8 kg/m2, respectively. The type of pouch was classified into one of four groups that included: concentric pouch dilatation (CP, n=4), eccentric pouch dilatation with the normal band angle (EPA1, n=3), eccentric pouch dilatation with the horizontal band (EPA2, n=2), eccentric pouch dilatation with downward migration of the band (EPA3, n=2), and eccentric pouch dilatation with posterior band slippage (EPP, n=3). The median time interval between primary band surgery and diagnosis of pouch dilatation was 12.5 months (range, one day to 28 months). Principal symptoms were as follows: food intolerance (11/14, 79%), nocturnal reflux (9/14, 64%), frequent vomiting (12/14, 86%), epigastric pain (11/14, 79%), and, infrequently, loss of restriction (2/11, 14%). BMI and percentage of excess weight loss (EWL) of patients was 25.1±3.6 kg/m2 and 87.2±29.1%, respectively, except for two patients with posterior slippage during the immediate postoperative period (Table 1). Conservative management was initially attempted for all patients, except for two cases of posterior slippage (cases 12 and 14) that were corrected by immediate band replacement. All four patients (cases 1-4) with CP showed a good response to conservative management. After a median follow-up period of three months (range, 1-6 months), no recurrence of clinic-radiologic signs and symptoms was observed. However, two patients (cases 2 and 4) experienced a slight increase in body weight during the follow-up period. Conservative management was also administered to seven patients with eccentric pouch dilatation with anterior slippage (EPA1-3). Three EPA1 patients (cases 5-7) showed a positive response to conservative management without operative intervention. Although no recurrence of clinico-radiologic signs and symptoms were observed in these patients, band adjustment with proper saline volume was not possible, and they experienced regain of weight during the follow-up period. Among the other four patients (EPA2/3), band replacement was necessary in two patients (cases 8 and 10). These two patients did not respond to initial conservative management. Severe night time reflux and postprandial epigastric discomfort were the primary reasons for revisions; the other two patients (cases 9 and 11) recovered well after conservative management without discomfort. All three EPP patients (cases 12-14) underwent reoperation. Evidence of lesser sac penetration with redundant posterior fundus was observed during revision in two patients (cases 12 and 14). Comparison of pre- and post-intervention data of each group (conservative treatment group vs. surgical treatment group) is summarized in Table 2. In our practice, we used the gastric plication technique below the band in 27 patients (Fig. 1). Among 27 patients, only one patient was diagnosed as having concentric pouch dilatation, and there was no single episode of eccentric pouch dilatation. Their median follow-up period was 13 months (range, 4-28 months).
Pouch enlargement and band slippage have been reported as the most common complications after adjustable gastric band placement.13,14 With the lack of a standard definition of pouch dilatation and slippage, many studies have reported on slip and pouch dilatation together as a single complication.15 However, for a better understanding of the pathophysiology, pouch dilatation and band slippage have been described as separate entities in recent literature.13,16 In our study, the incidence of pouch dilatation (11.1%) was rather high when compared to other studies covering this topic.15 Our practice started in early 2009; therefore, we skipped the era of the perigastric technique. However, we still observed three cases of posterior slippage during our study period. Two patients were within the first ten patients in our practice, and, band placement was not adequate in one patient due to a scar from a previous operation. In our study, the complications were divided into concentric pouch without band slippage (3.2%), eccentric pouch with anterior slippage (5.6%), and eccentric pouch with posterior slippage (2.4%); thus, complications reported in the present series were comparable with those reported in other studies.15 Occurrence of pouch dilatation and slippage is associated with various factors including surgical technique, patients' compliance with the procedure, and postoperative management. Technical problems associated with anterior slippage include rupture of stitches, and sutures that include only perigastric fat, which will become loosened with the passage of time. For gastrogastric sutures, there are several randomized controlled trials showing conflicting results.17-19 However, it is clear that improper fixation of the band or rupture of stitches can lead to occurrence of slippage.20,21 Presence of a concentric pouch is not dangerous, and usually results from over-inflation of the band or poor eating habits of the patients.16,22 Therefore, management of concentric pouch dilatation is rather straightforward; total or near total unfill and gradual refill. In all patients with CP from our study, pouch size was normalized immediately after unfill. If diagnosed early, this type of pouch dilatation may be resolved with deflation of the band.23 By contrast, management of an eccentric pouch was more complicated. Moser, et al.13 reported an association of pouch enlargement with band slippage, and it is unlikely that these patients would benefit from conservative treatment. Eccentric pouch dilatation on UGI barium swallow study, which is most often a late complication following LAGB, is caused by slippage of the band,23 and is associated with a more serious pouch abnormality associated with band dislocation. In our study, EPA1 patients showed early band slippage. Radiologically, the angle of the band was normal. However, the configuration showed a somewhat "ring like" pattern rather than a normal slit like shape. Over-tightening of the band with frequent vomiting was the most probable cause for all three patients. In order to prevent this complication, early or rapid band adjustment should be avoided. Because the gastric wall inside the band (gastric herniation) already exists, even a small addition of saline in the band can cause outlet obstruction. We recommend a very small and gradual band adjustment under fluoroscopic guidance in these patients. Patients in the EPA2 group showed a more horizontal band angle and a more eccentric pouch. Usually, this type of pouch is a consequence of the chronic process, which is found later, compared to EPA1. The two patients in our study already achieved their ideal body weight. However, one of these patients underwent band replacement for resistance of severe night time reflux to conservative management. Patients in the EPA3 group presented with acute and more severe symptoms. This type of pouch is usually associated with full-blown band dislocation. For initial management, immediate total unfilling of the band was always preferred. If symptoms persist, and the pouch is not normalized radiologically, revision should be performed. Various rates of success, from 21.8-82.7%, of revision surgery for the management of a slipped band, such as repositioning or replacement, have been reported.20,24-27 In order to avoid possible complications, such as fistula, leakage, and bleeding, careful dissection is necessary. Use of the ultrasonic shear device was very useful to minimize bleeding. We also used anterior and posterior plications in order to stabilize the band during band replacement. Due to extensive adhesion around the pouch, we have not performed simpler gastric reduction as a revision option for management of a slipped band. Keidar, et al.20 reported on four cases of recurrent slippage (10%) among 40 patients who underwent reduction for management of band slippage. Manganiello, et al.12 also reported six cases of recurrent slippage (55%) among 11 patients who underwent reduction. In general, rupture of a gastrogastric suture can result in anterior slippage of the band. In addition, potential space within the band resulting from reduced perigastric fat due to weight loss may cause slippage. Srikanth, et al.28 suggested that complete closing of the space underneath the band could prevent herniation of the anterior stomach through the band. From the beginning of our practice, we have anecdotally performed anterior plication beneath the band during performance of primary banding surgery with the hope of further stabilizing the band to prevent slippage and pouch dilatation. A total of 27 patients underwent this plication procedure during the study period. With the exception of one patient with a concentric pouch, these patients have not shown eccentric pouch formation. In fact, the portion of the fundus redundant below the band caused the partial fundal prolapsed, thus causing anterolateral band slippage. For some patients, the redundant portion is in the anterior gastric wall, yet for others, the redundant portion is in the posterolateral gastric wall. Band fixation with anterolateral gastro-gastric plication stitches therefore cannot prevent band slippage and pouch dilatation for all the banded patients.
In summary, pouch dilatation with or without slippage is a long term complication in LAGB patients; the percentage observed in our three-year study was 11.1%. Except for posterior slippage, these complications show an association with postoperative management, such as over tightening of the band or poor compliance among patients. Gradual band adjustment with strict follow-up and continued dietary consultation will prevent or minimize occurrence of these complications. From a technical point of view with consideration of accurate pars flaccida techniques, accurate seromuscular bites are very important because inaccurate gastrogastric fixation can be loosened with time (stitch burst), and eventually make the portion of the fundus redundant below the band. Removal of the fat pad on the cardia with coagulation greatly facilitates the accurate seromuscular bites. In addition, adequate wrapping also stabilizes the gastric band and minimizes the fundal prolapse, thus avoiding slippage. In the case of acute posterior slippage/pouch dilatation (EPP), we usually perform emergent surgery right after band deflation because conservative treatment has rarely been proved beneficial. In the case of anterior slippage/pouch dilatation (EPA), initial management should achieve complete band deflation. Based on our experience, acute anterior slippage/pouch dilatations (with significant clockwise rotation of band, EPA) often responds well to conservative management, otherwise it can cause persistent symptoms, even after complete band deflation. In the latter case, band revision should be performed (usually within 1-2 weeks) to prevent further catastrophes such as dehydration, gastric wall ischemia, and peritonitis. For chronic anterior slippage/pouch dilatations (EPA1/2), reflux is the main symptom, but can be managed by band adjustment. However, frequently, patients regain their body weight with their loosened band. In that case, we perform an elective surgery for band revision. The proper timing of this elective surgery should be discussed with the patient. As seen in three EPA1 cases and one EPA2 case, early/chronic band slippage that is detected only by radiography with minimal reflux symptom, usually can be managed conservatively. Band replacement into the new retrogastric tunnel was effective as a revision option in those who failed to respond to conservative management.
Figures and Tables
Fig. 1
Oblique plication technique. After placement of three gastrogastric sutures above the band, we placed four or five seromuscular stitches of 2-0 Ethibond® (Ethicon, Somerville, NJ, USA) on the anterolateral gastric wall, thereby enabling further stabilization of the band and gastric wall.

Fig. 2
Concentric pouch dilatation. Normal band position and normal band angle were noted. The pouch was dilated concentrically. The pouch appears to have migrated to the intrathoracic level, suggesting the presence of a coexisting hiatal hernia.
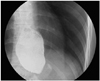
Fig. 3
Upper GI study of eccentric pouch dilatation. (A) EPA1, eccentric pouch with a normal band angle with a ring-like band configuration. Radiologically, this type is early anterior slippage. (B) EPA2, eccentric pouch with a more horizontal band angle. This type of dilatation usually results in a progressive chronic symptom of acid reflux. (C) EPA3, eccentric pouch with excessive clockwise rotation of the band. This type of dilatation usually manifests as acute, total food intolerance with severe reflux and epigastria pain. (D) EPP, eccentric pouch with posterior band slippage. This type of dilatation is associated with use of poor surgical techniques (e.g. entering the lesser sac with a redundant posterior gastric wall). Arrow indicate outlines of the dilated pouch above band.

Fig. 4
Laparoscopic non-destructive removal of the band and its repositioning at a proper level in an EPA3 patient (patient #10). (A) In patients with pouch enlargement with severe reflux, a variable degree of hiatal hernia was usually observed, and we performed concomitant repair using figure of eight sutures of the anterior crura muscle. Plicated neofundus was anchored to the crural muscle fascia (short arrow), and gastogastric suture was also performed (long arrow). Asterisk: newly formed pouch. (B) Repositioning of the gastric band through the newly formed retrogastric tunnel above the previous band position (circular area). Anterior plication of the gastric wall below the band was performed (arrow). Preop (C) and postop (D) gastrograffin swallow study showed that the band angle and pouch shape (arrows) were normalized.

Table 1
Demographics, Clinico-Radiologic Features, Management, and Follow-Up Data of 14 Patients Who Demonstrated Pouch Dilatation with/without Band Slippage

FI, food intolerance; NR, night time reflux; FV, frequent vomiting; EP, epigastric pain; LR, loss of restriction; CP, concentric pouch dilatation; BMI, body mass index; Tx, treatment; FU, follow-up period; EWL, excess weight loss.
*Follow-up period after treatment of pouch dilatation/slippage.
†Calculated with the ideal BMI of 23 kg/m2.29
References
1. Tolonen P, Victorzon M, Mäkelä J. 11-year experience with laparoscopic adjustable gastric banding for morbid obesity--what happened to the first 123 patients? Obes Surg. 2008; 18:251–255.

2. Himpens J, Cadière GB, Bazi M, Vouche M, Cadière B, Dapri G. Long-term outcomes of laparoscopic adjustable gastric banding. Arch Surg. 2011; 146:802–807.

3. Suter M, Calmes JM, Paroz A, Giusti V. A 10-year experience with laparoscopic gastric banding for morbid obesity: high long-term complication and failure rates. Obes Surg. 2006; 16:829–835.

4. Stroh C, Hohmann U, Schramm H, Meyer F, Manger T. Fourteen-year long-term results after gastric banding. J Obes. 2011; 2011:128451.

5. Kriwanek S, Schermann M, Ali Abdullah S, Roka R. Band slippage--a potentially life-threatening complication after laparoscopic adjustable gastric banding. Obes Surg. 2005; 15:133–136.

6. Hofer M, Stöllberger C, Finsterer J, Kriwanek S. Recurrent aspiration pneumonia after laparoscopic adjustable gastric banding. Obes Surg. 2007; 17:565–567.

7. Yoon CI, Pak KH, Kim SM. Early experience with diagnosis and management of eroded gastric bands. J Korean Surg Soc. 2012; 82:18–27.

8. Seo WJ, Pak KH, Kim SM. Novel method for port implantation in lap-band surgery--transumbilical subfascial port implantation. J Laparoendosc Adv Surg Tech A. 2012; 22:254–258.

9. Fielding GA, Allen JW. A step-by-step guide to placement of the LAP-BAND adjustable gastric banding system. Am J Surg. 2002; 184:26S–30S.

10. Ponce J, Paynter S, Fromm R. Laparoscopic adjustable gastric banding: 1,014 consecutive cases. J Am Coll Surg. 2005; 201:529–535.

11. Vertruyen M. Repositioning the Lap-Band for proximal pouch dilatation. Obes Surg. 2003; 13:285–288.

12. Manganiello M, Sarker S, Tempel M, Shayani V. Management of slipped adjustable gastric bands. Surg Obes Relat Dis. 2008; 4:534–538.

13. Moser F, Gorodner MV, Galvani CA, Baptista M, Chretien C, Horgan S. Pouch enlargement and band slippage: two different entities. Surg Endosc. 2006; 20:1021–1029.

14. Sherwinter DA, Powers CJ, Geiss AC, Howard M, Warman J. Posterior prolapse: an important entity even in the modern age of the pars flaccida approach to lap-band placement. Obes Surg. 2006; 16:1312–1317.

15. Egan RJ, Monkhouse SJ, Meredith HE, Bates SE, Morgan JD, Norton SA. The reporting of gastric band slip and related complications; a review of the literature. Obes Surg. 2011; 21:1280–1288.

16. Brown WA, Burton PR, Anderson M, Korin A, Dixon JB, Hebbard G, et al. Symmetrical pouch dilatation after laparoscopic adjustable gastric banding: incidence and management. Obes Surg. 2008; 18:1104–1108.

17. Avsar FM, Sakcak I, Yildiz BD, Cosgun E, Hamamci EO. Is gastro-gastric fixation suture necessary in laparoscopic adjustable gastric banding? A prospective randomized study. J Laparoendosc Adv Surg Tech A. 2011; 21:953–956.

18. Fried M, Dolezalova K, Sramkova P. Adjustable gastric banding outcomes with and without gastrogastric imbrication sutures: a randomized controlled trial. Surg Obes Relat Dis. 2011; 7:23–31.

19. Lazzati A, Polliand C, Porta M, Torcivia A, Paolino LA, Champault G, et al. Is fixation during gastric banding necessary? A randomised clinical study. Obes Surg. 2011; 21:1859–1863.

20. Keidar A, Szold A, Carmon E, Blanc A, Abu-Abeid S. Band slippage after laparoscopic adjustable gastric banding: etiology and treatment. Surg Endosc. 2005; 19:262–267.

21. O'Brien PE, Dixon JB. Laparoscopic adjustable gastric banding in the treatment of morbid obesity. Arch Surg. 2003; 138:376–382.
22. Wiesner W, Schöb O, Hauser RS, Hauser M. Adjustable laparoscopic gastric banding in patients with morbid obesity: radiographic management, results, and postoperative complications. Radiology. 2000; 216:389–394.

23. Carucci LR, Turner MA, Szucs RA. Adjustable laparoscopic gastric banding for morbid obesity: imaging assessment and complications. Radiol Clin North Am. 2007; 45:261–274.

24. Chevallier JM, Zinzindohoué F, Elian N, Cherrak A, Blanche JP, Berta JL, et al. Adjustable gastric banding in a public university hospital: prospective analysis of 400 patients. Obes Surg. 2002; 12:93–99.

25. Suter M, Bettschart V, Giusti V, Heraief E, Jayet A. A 3-year experience with laparoscopic gastric banding for obesity. Surg Endosc. 2000; 14:532–536.

26. Suter M. Laparoscopic band repositioning for pouch dilatation/slippage after gastric banding: disappointing results. Obes Surg. 2001; 11:507–512.

27. Foletto M, Bernante P, Busetto L, Pomerri F, Vecchiato G, Prevedello L, et al. Laparoscopic gastric rebanding for slippage with pouch dilation: results on 29 consecutive patients. Obes Surg. 2008; 18:1099–1103.

28. Srikanth MS, Oh KH, Keskey T, Rumbaut R, Fox SR, Fox ER, et al. Critical extreme anterior slippage (paragastric Richter's hernia) of the stomach after laparoscopic adjustable gastric banding: early recognition and prevention of gastric strangulation. Obes Surg. 2005; 15:207–215.

29. WHO Expert Consultation. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004; 363:157–163.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download