Abstract
Purpose
Cefaclor is widely prescribed for various infectious diseases. As its consumption increases, the number of hypersensitivity reactions to cefaclor has increased. This study aimed to evaluate the immunologic findings of immediate hypersensitivity to cefaclor.
Materials and Methods
We enrolled 47 patients with immediate hypersensitivity to cefaclor from Ajou University Hospital and Asan Medical Center. Serum specific IgE, IgG1, and IgG4 antibodies to cefaclor-human serum albumin (HSA) conjugate were measured by enzyme-linked immunosorbent assay (ELISA).
Results
The most common phenotype was anaphylaxis (Group I, 78.7%), followed by urticaria (Group II, 21.3%). The detection of specific IgE, IgG1, and IgG4 to cefaclor-HSA conjugate by ELISA tended to be higher in Group I (40.5%, 41.7%, 21.6%) than in Group II (20.0%, 20.0%, 0%) with no statistical significance. Significant associations were found between specific IgE and IgG1 or IgG4 (p<0.001, p=0.019). ELISA inhibition tests showed significant inhibitions by both free cefaclor and cefaclor-HSA conjugate. For basophil activation tests in patients having no specific IgE antibody, the CD63 expression level on basophils increased with incubations of free cefaclor.
Cephalosporins are more widely prescribed than penicillin to treat common infections due to their broad spectrum of antibacterial activity and low toxicity profiles.1,2,3 However, the number of hypersensitivity reactions to cephalosporins (including anaphylaxis) has increased with their use.1,3 Depending on the onset of symptom development, hypersensitivity reactions to cephalosporins are divided into immediate and delayed reactions. The clinical symptoms of immediate hypersensitivity reactions to cephalosporins (usually within 1 hour after exposure) can affect various organs; however, the two main entities usually recognized are urticaria and anaphylaxis.4
Cephalosporin hypersensitivity reactions to cefaclor are the most common and have been recognized since early clinical use.5 A study on 1170 children with immediate reactions to penicillin and cephalosporins reported that cefaclor showed the highest frequency (29.2%) of positive reactions to both skin prick tests and challenges.6 Immediate hypersensitivity reactions are generally considered IgE mediated; however, cefaclor allergies with documented IgE mediated reactions have been estimated at up to 80%,7,8 and other possible immunologic mechanisms are unclear. This study investigated the immunologic findings of immediate hypersensitivity to cefaclor in order to further understand the pathogenic mechanisms of cefaclor-induced hypersensitivity.
We retrospectively enrolled 47 patients with a history of immediate reactions to cefaclor from Ajou University Hospital and Asan Medical Center. In addition, we enrolled healthy controls from the general population. Patients' clinical data including age, gender, history of allergic diseases, and drug allergies were obtained from a review of electronic medical records by clinicians. Healthy controls were non-atopic and had no medical history of allergic disease or drug allergy. Patients with immediate hypersensitivity reactions to cefaclor were defined by a certain clinical history with or without serum specific IgE to cefaclor using the ImmunoCAP system (Thermo Fisher Scientific Inc., Uppsala, Sweden). Study subjects were divided into two groups according to the major manifestations of hypersensitivity reactions (Group I: patients with anaphylaxis to cefaclor; Group II: patients with urticaria or angioedema). Anaphylaxis was diagnosed based on the definitions of the National Institute of Allergy and Infectious Disease and the Food Allergy and Anaphylaxis Network.9 In addition, severity was classified by Simon's grading system.10 A skin prick test (SPT) was performed with 55 common aeroallergens (Bencard Co., Bredford, UK). Normal saline and 1 mg/mL histamine were used as negative and positive controls, respectively. An SPT was interpreted as positive if a wheal larger than 3 mm with surrounding erythema appeared 15 minutes after exposure. Total serum IgE and aeroallergen-specific IgE were measured using the ImmunoCAP system (Thermo Fisher Scientific Inc.). Atopy was defined by a positive SPT result or a high level of specific IgE to common aeroallergens >0.35 kU/L. Commercially available assays for cefaclor specific IgE were performed for all patients (Thermo Fisher Scientific Inc.). SPTs and intradermal skin tests (IDTs) were done with cefaclor (0.1, 1, and 10 mg/mL) on the volar forearm skin. Patients were initially tested using SPTs. When SPT responses were negative, 0.02 mL of serially diluted reagents in sterile 0.9% NaCl was injected intradermally. To find the cross-reactivity between cefaclor and other ß-lactam (BL) antibiotics, specific IgE in vitro assays for penicilloyl G, penicilloyl V, amoxicilloyl, and ampicilloyl were tested (ImmunoCAP system, Thermo Fisher Scientific Inc.). We performed an oral provocation test (OPT) with a therapeutic dose of cefaclor (250 mg). Subjects were carefully monitored during the test and cardiopulmonary resuscitation equipment was immediately available. The present study was approved by the Institutional Review Board of Ajou University Hospital and Asan Medical Center (AJIRB-GEN-GEN-09-140). All study participants provided informed written consent.
Serum samples from 47 patients and 29 controls were collected and stored at -20℃. To detect serum specific IgE, IgG1, and IgG4 antibodies to cefaclor, cefaclor-human serum albumin (HSA) conjugate was prepared, and an enzyme-linked immunosorbent assay (ELISA) was conducted as described previously.11,12 Microplates (Corning, New York, USA) were coated with cefaclor-HSA conjugate (10 µg/mL per well), and incubated with the sera of patients and controls. Goat anti-human IgE antibody (Kirkegaard & Perry laboratories Inc., Gaithersburg, MD, USA), alkaline phosphate-conjugated rabbit anti-goat IgG antibody (ReserveAPTM; Kirkegaard & Perry Laboratories Inc., Gaithersburg, MD, USA), and p-nitrophenyl phosphate (St. Louis, MO, USA) substrate were added to each well in consecutive order. Biotinylated anti-human IgG1 antibody (Sigma-Aldrich, St. Louis, MO, USA) and biotinylated anti-human IgG4 antibody (Sigma-Aldrich) were used for the detection of serum specific IgG1 and IgG4 to cefaclor-HSA conjugate. The positive cutoff value for the ELISA was determined as the mean plus three times the standard deviation (SD) for the absorbance values of healthy controls.
ELISA inhibition tests were performed (as described previously) to evaluate the specificities of specific IgE and IgG antibodies and possible cross-reactivities with other BL antibiotics.11,12 Binding specificities were confirmed by ELISA with serial additions of increasing concentrations (1-100 µg/mL) of cefaclor-HSA conjugate and free cefaclor using serum samples from patients who had high levels of specific IgE, IgG1, or IgG4 antibodies to cefaclor. ELISA inhibition tests with the addition of free BL antibiotics (including cefaclor, penicillin, ampicillin, amoxicillin, and cephalexin) assessed cross-reactivities with cefaclor. The results of the inhibition tests were expressed as a percentage: % inhibition=100×[1-(absorbance with inhibitor/absorbance without inhibitor)].2
For the functional in vitro test for allergic reactions to cefaclor, a basophil activation test (BAT) was performed using flow cytometry (FACScantoII; BD Immunocytometry Systems, San Jose, CA, USA), using CD63 antibody according to previously described methods.13 Patients' basophils were incubated with cefaclor at dilutions of 1.6×10-3, 1.6×10-2, and 1.6×10-1 mg/mL for 30 minutes. BATs were conducted for two healthy controls under the same condition to exclude a nonspecific activation of basophil. The stimulation index (SI) was calculated as follows: SI=percentage of activated basophils after stimulation with free cefaclor/percentage of basophils with no free cefaclor stimulation.14
The demographic and clinical findings of study subjects are summarized in Table 1. The number of female subjects was 31 (66.0%) and the mean age was 40.28±13.23 years, ranging from 13 to 70 years. The most common phenotype of immediate hypersensitivity to cefaclor was anaphylaxis (Group I, 37 of 47 subjects, 78.7%), followed by urticaria with or without angioedema (Group II, 10 of 47 subjects, 21.3%). Thirty-five patients (76.1%) had underlying chronic diseases, such as hypertension, diabetes mellitus, and liver disease. Nineteen (51.4%) were atopic and 22 (47.8%) had a history of respiratory allergic diseases, such as bronchial asthma or allergic rhinitis. Among the study subjects, 17 (36.2%) had a history of other drug allergies besides cefaclor hypersensitivity. The mean serum total IgE level was 305.93±334.67 kU/L. Forty-two subjects (89.4%) had high serum specific IgE to cefaclor (>0.35 kU/L) measured by the ImmunoCAP system (Thermo Fisher Scientific Inc.), and the prevalence of serum specific IgE to penicillin (penicilloyl G or penicilloyl V) and aminopenicillin (amoxicilloyl or ampicilloyl) was 11.9% and 4.7%, respectively. Of the 47 patients, only 11 (23.4%) were orally challenged with cefaclor. All patients displayed positive responses to OPTs. Of the 13 patients who underwent skin tests with cefaclor extracts, 8 (61.5%) showed positive responses to SPT or IDT.
The serum levels of specific IgE, IgG1, and IgG4 to cefaclor-HSA conjugate are shown in Fig. 1. The prevalence of serum specific IgE, IgG1, and IgG4 antibodies in sera of the study subjects was 36.2%, 37.0%, and 17.0%, respectively. The ELISA inhibition tests for specific IgE, IgG1, and IgG4 showed significant inhibitions with the addition of both free cefaclor and cefaclor-HSA conjugate in dose-dependent manners (Fig. 2).
All clinical parameters and immunological findings were compared according to the major clinical presentations, anaphylaxis (Group I) and urticaria (Group II). Data shown in Table 2 indicated no significant differences in baseline clinical characteristics such as age, gender, and atopy between the two groups (p>0.05). No significant difference was found in the prevalence of serum specific IgE to cefaclor by ImmunoCAP for the two groups (Group I: 89.2%, Group II: 90%). The detection of serum specific IgE, IgG1, and IgG4 to cefaclor-HSA conjugates by ELISA tended to be higher in Group I (40.5%, 41.7%, and 21.6%) than in Group II (20%, 20%, and 0%); however, the differences were not statistically significant. The detection of serum specific IgG4 to cefaclor-HSA conjugate by ELISA was observed only in Group I. No patient had a high serum specific IgG4 without serum specific IgE. Two patients (5.6%) in Group I showed a high level only for specific IgG1 to cefaclor-HSA conjugate with no specific IgE or IgG4 levels detectable by ImmunoCAP and ELISA.
Of the 47 patients tested, 42 patients (89.4%) showed high serum specific IgE to cefaclor by ImmunoCAP. Clinical features compared by ImmunoCAP for patients with and without a high specific IgE showed no significant differences in gender, atopy rate, associated allergic diseases, peripheral eosinophil count, and the results of oral provocation tests (p>0.05) (Table 3). The mean age was significantly younger in patients with high serum specific IgE (38.88±12.61 years) than in those without (52.0±13.8 years). Serum total IgE was significantly higher in the positive group than in the negative group (336.22±341.43 kU/L, 57.58±88.27 kU/L, respectively; p<0.001). The prevalence of serum specific IgG1 and IgG4 antibodies was not significantly different between the two groups (36.6% and 40.0% for specific IgG1, 19.0% and 0% for specific IgG4, p>0.05). However, serum specific IgG4 to cefaclor by ELISA was detected only in patients with high specific IgE to cefaclor by ImmunoCAP.
Among the 47 patients, 17 (36.2%) had high serum specific IgE to cefaclor-HSA conjugate by ELISA and were lower than those measured by the ImmunoCAP system. When the clinical features were compared according to the presence of serum specific IgE to cefaclor by ELISA, significant associations were found between specific IgE and both specific IgG1 (p<0.001) and specific IgG4 (p=0.019). The prevalence of serum specific IgG1 and IgG4 was significantly higher in patients with a high specific IgE detected by ELISA (81.3%, 35.3%) than in those without specific IgE (13.3%, 6.7%). Patients with a high serum specific IgE by ELISA were younger (31.94±7.19 years) than those without specific IgE (45.00±13.61 years). Table 4 shows no significant differences in gender, atopy rate, associated allergic diseases, peripheral eosinophil counts, serum total IgE, results of skin tests, and OPTs (p>0.05).
The overall prevalence of specific IgE to penicillin and aminopenicillin was 11.9% and 4.7%, respectively, with no significant differences in the prevalence of penicillin and aminopenicillin according to the presence of serum specific IgE to cefaclor by ImmunoCAP [13.5%, 0% (penicillin); 5.3%, 0% (aminopenicillin); p>0.05] and ELISA [7.7%, 13.8% (penicillin); 0%, 6.7% (aminopenicillin); p>0.05]. Moreover, IgE ELISA inhibition tests performed by adding penicillin, aminopenicillin, and cephalexin to the sera of two patients with a high specific IgE to cefaclor resulted in significant inhibitions for free cefaclor. Minimal inhibition was noted with cephalexin in one patient; however, no inhibitions were found with the addition of free forms of penicillin and aminopenicillins (ampicillin and amoxicillin) (Fig. 3).
We performed BATs since 20% of patients did not have serum specific IgE (using either ImmunoCAP or ELISA) to evaluate a possible mechanism of direct basophil activation. Basophils were collected from patients with no specific antibodies in their serum. Following incubation with free cefaclor, CD63 expression level on the blood basophils of the subject increased from 5.3% (spontaneous CD63 expression) to 10.0% (the highest CD63 expression; SI=1.9), whereas the same test on basophils collected from normal controls did not increase to cefaclor, but decreased (Fig. 4).
Allergy to BL antibiotics is still the most frequent cause of antibiotics allergies. Among BL antibiotics, cephalosporins have become the most common cause of systemic IgE-dependent reactions since the use of cephalosporins has replaced penicillin use.15 In addition, cefaclor (a second generation cephalosporin) was recently reported as the second most frequently prescribed systemic antibiotic in Korea.16 Due to oral preparation, cefaclor is more commonly prescribed for children and those in outpatient clinics. Subsequently, hypersensitivity reactions to cefaclor are more often reported as rashes, urticaria, angioedema, serum sickness-like reactions, myocarditis, and even anaphylaxis.5,8,11,17,18,19 Until a few years ago, anaphylaxis of cefaclor was recognized only as a rare case report;5,20 however, numerous anaphylaxis reactions to cefaclor have been recently reported.8,11,17 In general, the risk of anaphylaxis to cephalosporins appears low, with a reported incidence of 0.001-0.1%; however, the incidence of anaphylaxis to cefaclor was reported as being relatively higher (0.01%) than other cephalosporins in adults, as well as in children.17,21,22,23 The incidence of immediate hypersensitivity to cefaclor is increasing; however, the underlying mechanisms of cefaclor hypersensitivity remains clarified. In this study, we evaluated the clinical and immunologic findings of patients with immediate hypersensitivity to cefaclor.
The most common clinical manifestation of cefaclor hypersensitivity was reported as skin lesions (e.g., urticaria, angioedema, and maculopapular exanthema) ranging from 1-2.8%.5,24 In a study of hypersensitivity to cephalosporin in children from allergy units, cefaclor was the most common cephalosporin responsible for immediate reactions, representing as anaphylaxis (64%) and urticaria with or without angioedema (37%).19 In a study regarding Korean adult patients with immediate hypersensitivity to cefaclor, the most frequent manifestation was anaphylaxis (76%), followed by urticaria and angioedema (24%).8 In the present study, the most common clinical feature of immediate hypersensitivity to cefaclor was anaphylaxis (78.7%), followed by urticaria with or without angioedema (21.3%), which is comparable to the previously mentioned reports. However, the higher frequency of anaphylaxis in the above studies, which contrasts with other previous reports, could be attributed to patient recruitment from university hospitals or allergic units.5,21,24 Anaphylaxis has become an increasing health burden in developed countries and a worldwide problem, contributing to multiple studies and surveys of anaphylaxis over the last decade.25 Anaphylaxis to cefaclor is more recognized now, and past anaphylaxis rates seem have been underestimated.26 Commonly associated symptoms of anaphylaxis are skin symptoms such as generalized urticaria, itching, and angioedema. Following skin symptoms, respiratory symptoms are the second most frequent features, such as shortness of breath, dyspnea, and wheezing.8,11,17 In the present study, all subjects with anaphylaxis presented with urticaria. Therefore, it is notable that anaphylaxis reactions to cefaclor are not rare and initially present with urticaria.
Various risk factors are related to the clinical expression of drug allergies. Drug-specific risk factors include dose, route of administration, and treatment duration. Host risk factors include age, gender, atopy, specific genetic polymorphism, and comorbidity.27 In immediate hypersensitivity to penicillin, patients between the ages of 20 and 49 have the highest risk.28 In the present study, the mean age was 40.28±13.23 years old. In a retrospective study of anaphylaxis, underlying disease (especially cardiovascular disease) was reported as a risk factor for severe anaphylaxis.29 In our study, patients with anaphylaxis showed a higher frequency of underlying disease; however, there was no significant difference. Generally, individuals who have any history of allergic reactions to the same drugs or to cross-reactive drugs convey a high risk. In the present study, 12.8% had a history of antibiotic allergy (13.5% in anaphylaxis, 10.0% in urticaria). In severe and fatal penicillin anaphylaxis, atopy is a considerable risk factor.28 However, the atopy rate was 51.4% for all subjects in the present study with no differences between anaphylaxis and urticaria groups; therefore atopy is not a predisposing factor for cefaclor-induced anaphylaxis. Known risk factors for drug allergies were not significant in the present study; consequently, age, atopy, and underlying disease should be considered before cefaclor prescription.
Diagnosis of many drug allergy cases is based on presumption due to the lack of available specific confirmatory tests. As a method of diagnosing immediate hypersensitivity to cephalosporin, skin tests with cephalosporin, in vitro tests of solid-phase immunoassays (radioallergosorbent test, ELISA) to detect specific IgE to cephalosporin, flow cytometry assessing drug-induced basophil activation by means of CD63 or CD203c expression, and confirmative oral challenge tests can be available.4 In immediate hypersensitivity to penicillin, the chemical structure permits the identification of allergenic determinants. Penicillin skin testing is a reliable method to evaluate IgE-mediated hypersensitivity to penicillin.3 However, unlike penicillin, exact allergenic determinants of cephalosporins have not been identified. The validity of a skin test for cephalosporin hypersensitivity remains controversial.3,30,31 Results ranged from 0.3% to 69.7% in studies that attempted to determine the sensitivity of a cephalosporin skin test.3 In cefaclor hypersensitivity, the rate of positive skin test results was 29.2%.32 The present study performed skin tests on anaphylaxis patients, and the positive rate of skin test results was 61.5%. Using a nonirritating concentration of culprit cephalosporin, a positive reaction from the skin test suggests the presence of the cephalosporin-specific IgE antibody. However, the negative predictive value of the cephalosporin skin test is not yet known, and a negative response to a skin test cannot rule out cephalosporin immediate hypersensitivity mediated by IgE. Cephalosporins are structurally and pharmacologically analogous to penicillin, having a side chain in C7 (R1) and different substituent (R2) in C3.1,33 Low molecular weight cephalosporin is covalently bound to high molecular weight carrier proteins, after which the newly developed drug-protein complexes induce an immunologic response. However, insufficient knowledge about degradation products and how to conjugate with a carrier protein remains an impediment in developing in vitro tests for cephalosporin hypersensitivity.27 Cefaclor is composed of an aminobenzyl (R1) side chain and Cl at R2, of which the structure is clearly simple; consequently, there was earlier development of an immunoassay to detect specific IgE to cefaclor.33 An in vitro test of fluorescent enzyme immunoassays (FEIA) (ImmunoCAP, Thermo Fisher Scientific Inc.) is available only for cefaclor among cephalosporin hypersensitivities. The simplicity of the R2 structure could be why the sensitivity (89.4%) of ImmuneCAP for cefaclor is higher compared to that (61.5%) of the skin test in the present study. The reported sensitivity rate of serum specific IgE to cefaclor by ImmunoCAP showed a range of 25-80%.8,11,17 The sensitivity gap could be attributable to the different timing of blood sampling and the different manufacturer. The results measured by ImmunoCAP showed a similar rate of over 75%. In the present study, the measurement of specific IgE by ImmunoCAP was mostly done at the visit to the hospital when hypersensitivity occurred or within 7 days. However, the detection rate of specific IgE to cefaclor differed between ImmunoCAP and ELISA (89.4% and 36.2%, respectively). There was no significant association for specific IgE between ImmunoCAP and ELISA. The principle of ELISA and FEIA is based on the solid phase to which the hapten conjugated to a carrier protein is bound covalently, which may be associated with the size and conformation of antigen molecules.7,34 The different haptens of cefaclor, detected by ImmunoCAP or ELISA, may be made from the same R side chain. However, specific IgE to cefaclor by ELISA was detected only in patients with a high specific IgE by ImmunoCAP. Therefore, for the diagnosis of immediate hypersensitivity to cefaclor, ImmunoCAP is more sensitive and useful than ELISA or a skin test. In our study, the overall documentation rate of specific IgE to cefaclor, combining serum specific IgE and skin tests, was 91.5%. The OPT is confirmatively diagnostic as a "gold standard;" however, the high-risk of this procedure outweighs the benefit of exact diagnosis. Two main reasons for performing an OPT are to exclude hypersensitivity in a less-likely history of drug hypersensitivity and to establish a firm diagnosis.30 Combined tests with a serum specific IgE and a skin test are required to increase the diagnostic rate of IgE-mediated hypersensitivity to cefaclor. OPTs should carefully be conducted only in selected patients with cefaclor hypersensitivity.
Allergy to cephalosporins was studied primarily in conjunction with penicillin allergy.1,3 Until the 1970s, frequent reports of anaphylaxis following the administration of first and second generation cephalosporins to patients with a history of penicillin allergy led to the conception of a high degree of cross-reactivity between penicillin and cephalosporins. In vitro studies with penicillin and cephalosporin showed a high degree of immunologic cross-reactivity (5-10%), which was believed to be due to the presence of the common BL ring.3,11,35,36,37,38 However, there have been less frequent relevant cross-relativities between penicillin and cephalosporins since 1980. The risk of cephalosporin hypersensitivity in penicillin allergy is recently reported at around 1-2%.3,39,40,41 Some studies showed that patients with hypersensitivity to particular cephalosporins could tolerate cephalosporins and suggested that the antibodies to the R-side chain rather than to the common BL ring are determining factors for the immune response to cephalosporins.3,42,43,44,45 Furthermore, several clinical studies have found that cross-reactivity between penicillin and first and second generation cephalosporins with identical or similar side chains ranges from 14-38%.1,43,46,47 In the present study, IgE ELISA inhibition results of cefaclor-HSA showed significant inhibitions with the addition of free cefaclor and minimal inhibitions with cephalexin (which shares an identical R-side chain with cefaclor). Therefore, cross-reactivity between cefaclor and cephalexin is not high. In addition, IgE ELISA inhibition tests of cefaclor-HSA with penicillin and aminopenicillin did not show any significant inhibitions. However, the detection rate of specific IgE to penicillin and aminopenicillin by ImmunoCAP were 11.6% and 4.5%, respectively. All patients with high specific IgE to penicillin and aminopenicillin were those with high specific IgE by ImmunoCAP. However, in two patients with a previous history of penicillin allergy, specific IgE to penicillin was not detected even though they showed positive ImmunoCAP results. The findings suggest that these patients have concurrent sensitivities to penicillin and aminopenicillin rather than immunologic cross-reactivity.
The present study measured serum specific IgG to cefaclor-HSA conjugate to find non-IgE mediated immediate hypersensitivity. In the present study, some patients had high specific IgG1 or IgG4 levels. The detection rates were higher in Group I (41.7% for IgG1, 21.6% for IgG4) than in Group II (20.0% for IgG1, 0% for IgG4). Notably, specific IgG4 was detected only in Group I patients. The mechanisms responsible for anaphylaxis have been investigated in animal models (in most cases) because anaphylaxis is a life-threatening medical emergency.48 Although the role of IgG in anaphylaxis has not been elucidated in humans, it has been suggested that IgG-mediated anaphylaxis occurs in mice, and specific IgG to drugs may contribute to human anaphylaxis reactions to drugs.45 In occupational allergic reactions, IgG antibody has been suggested to be associated with T helper2/B cells, induced IgE, and IgG4 production.12,49 As a result of chronic antigen exposure, IgG4 antibodies are produced in humans.50 However, the presence of drug specific IgG4 is often poorly correlated with immunopathologic mechanisms.27 In the present study, two patients (5.6%) in group I showed high specific IgG1 to cefaclor-HSA conjugate by ELISA with no specific IgE or IgG4. Further investigations will be needed to understand the role of specific IgG in the pathogenic mechanisms of cefaclor induced anaphylaxis. Meanwhile, anaphylaxis could be classified into two major mechanisms: "immunological" IgE-dependent or IgE-independent response.51 The latter includes basophil and mast cell degranulation without immunoglobulin. This mechanism is still poorly understood; however, anaphylaxis to various drugs, such as opiate medication, radiocontrast media, and ethanol, has revealed potential mechanisms.52,53,54,55 In the present study, BAT using basophils from a patient who did not have any specific IgE or IgG, showed an increased CD63 expression after incubation with cefaclor. In a previous study on fatal anaphylaxis to cephalosporins, six patients experienced allergic reactions after the first exposure of antibiotics.56 The findings suggest that cefaclor can induce anaphylaxis by a direct activation of basophils and mast cells; however, an extended study will be needed in a larger cohort.
In conclusion, the most common manifestation of immediate hypersensitivities to cefaclor was anaphylaxis, most of which was mediated by IgE antibody; however, we suggest a possible non-IgE mediated, but direct, basophil activation mechanism in a subset of cefaclor-induced anaphylaxis patients.
Figures and Tables
Fig. 1
Specific IgE (A), IgG1 (B), and IgG4 (C) bindings to cefaclor-human serum albumin by ELISA in sera from Group I (anaphylaxis) and II (urticaria) compared to normal controls (NC). The line, the cutoff value defined as mean+3 standard deviation of the absorbance value of controls. ELISA, enzyme-linked immunosorbent assay; HSA, human serum albumin.

Fig. 2
IgE (A), IgG1 (B), and IgG4 (C)-ELISA inhibition results of cefaclor-human serum albumin (HSA) coated wells with serial additions of cefaclor-HSA and free cefaclor. NHS, N-hydroxysulfosuccinimide; ELISA, enzyme-linked immunosorbent assay.

Fig. 3
IgE-ELISA inhibition results of cefaclor-human serum albumin coated wells with serial additions of free cephalosporins and penicillin and aminopenicillin in two different patients with cefaclor-hypersensitivity.

Fig. 4
The change in the expression of CD63 on basophils after incubation with free cefaclor in the patient (♦: without detection of specific IgE and IgG to cefaclor) and normal controls [atopic (■), non-atopic (▲)]. SI, stimulation index; NC, normal controls.

Table 2
Clinical Characteristics of the Study Subjects According to the Immediate Hypersensitivity
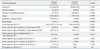
Group I, patients with anaphylaxis; Group II, patients with urticaria and or angioedema; ST, skin test; ND, not done; NA, not available; ELISA, enzyme-linked immunosorbent assay; +, positive result.
*Values are presented as mean±standard deviation.
†Penicillin means penicilloyl G and penicilloyl V.
‡Aminopenicillin means amoxicilloyl and ampicilloyl.
ACKNOWLEDGEMENTS
This research was supported by a grant of Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number: HI14C0065).
References
1. Moreno E, Macías E, Dávila I, Laffond E, Ruiz A, Lorente F. Hypersensitivity reactions to cephalosporins. Expert Opin Drug Saf. 2008; 7:295–304.

2. Kim JE, Kim SH, Jin HJ, Hwang EK, Kim JH, Ye YM, et al. IgE sensitization to cephalosporins in health care workers. Allergy Asthma Immunol Res. 2012; 4:85–91.

3. Dickson SD, Salazar KC. Diagnosis and management of immediate hypersensitivity reactions to cephalosporins. Clin Rev Allergy Immunol. 2013; 45:131–142.

4. Blanca M, Romano A, Torres MJ, Férnandez J, Mayorga C, Rodriguez J, et al. Update on the evaluation of hypersensitivity reactions to betalactams. Allergy. 2009; 64:183–193.

5. Grouhi M, Hummel D, Roifman CM. Anaphylactic reaction to oral cefaclor in a child. Pediatrics. 1999; 103:e50.

6. Atanasković-Marković M, Velicković TC, Gavrović-Jankulović M, Vucković O, Nestorović B. Immediate allergic reactions to cephalosporins and penicillins and their cross-reactivity in children. Pediatr Allergy Immunol. 2005; 16:341–347.

7. Gómez E, Torres MJ, Mayorga C, Blanca M. Immunologic evaluation of drug allergy. Allergy Asthma Immunol Res. 2012; 4:251–263.

8. Nam YH, Kim JE, Hwang EK, Jin HJ, Shin YS, Ye YM, et al. Clinical and immunologic evaluations of immediate hypersensitivity to cefaclor. Korean J Asthma Allergy Clin Immunol. 2011; 31:192–198.
10. Brown SG. Clinical features and severity grading of anaphylaxis. J Allergy Clin Immunol. 2004; 114:371–376.

11. Kim SH, Choi JH, Park HS. Heterogeneity of the IgE response to allergenic determinants of cefaclor in serum samples from patients with cefaclor-induced anaphylaxis. Ann Allergy Asthma Immunol. 2005; 94:700–704.

12. Nam YH, Kim JE, Kim SH, Jin HJ, Hwang EK, Shin YS, et al. Identifying genetic susceptibility to sensitization to cephalosporins in health care workers. J Korean Med Sci. 2012; 27:1292–1299.

13. Kim JH, An S, Kim JE, Choi GS, Ye YM, Park HS. Beef-induced anaphylaxis confirmed by the basophil activation test. Allergy Asthma Immunol Res. 2010; 2:206–208.

14. Kim MS, Cho YJ. Flow cytometry-assisted basophil activation test as a safe diagnostic tool for Aspirin/NSAID hypersenstivity. Allergy Asthma Immunol Res. 2012; 4:137–142.

15. Gruchalla RS, Pirmohamed M. Clinical practice. Antibiotic allergy. N Engl J Med. 2006; 354:601–609.
16. Sohn HS, Oh OH, Kwon JW, Lee YS. Higher systemic antibiotic consumption in a population of South Korea (2008-2009). Int J Clin Pharmacol Ther. 2013; 51:585–592.

17. Novembre E, Mori F, Pucci N, Bernardini R, Romano A. Cefaclor anaphylaxis in children. Allergy. 2009; 64:1233–1235.

18. Beghetti M, Wilson GJ, Bohn D, Benson L. Hypersensitivity myocarditis caused by an allergic reaction to cefaclor. J Pediatr. 1998; 132:172–173.

19. Romano A, Gaeta F, Valluzzi RL, Alonzi C, Viola M, Bousquet PJ. Diagnosing hypersensitivity reactions to cephalosporins in children. Pediatrics. 2008; 122:521–527.

20. Nishioka K, Katayama I, Kobayashi Y, Takijiri C. Anaphylaxis due to cefaclor hypersensitivity. J Dermatol. 1986; 13:226–227.

22. Annè S, Reisman RE. Risk of administering cephalosporin antibiotics to patients with histories of penicillin allergy. Ann Allergy Asthma Immunol. 1995; 74:167–170.
24. Kammer RB. Cefaclor in management of streptococcal pharyngitis, otitis media, and skin infections. Ann Otol Rhinol Laryngol Suppl. 1981; 90(3 Pt 3):79–81.

25. Patel DA, Holdford DA, Edwards E, Carroll NV. Estimating the economic burden of food-induced allergic reactions and anaphylaxis in the United States. J Allergy Clin Immunol. 2011; 128:110–115.

27. Joint Task Force on Practice Parameters. American Academy of Allergy, Asthma and Immunology. American College of Allergy, Asthma and Immunology. Joint Council of Allergy, Asthma and Immunology. Drug allergy: an updated practice parameter. Ann Allergy Asthma Immunol. 2010; 105:259–273.
28. Idsoe O, Guthe T, Willcox RR, de Weck AL. Nature and extent of penicillin side-reactions, with particular reference to fatalities from anaphylactic shock. Bull World Health Organ. 1968; 38:159–188.
29. Kim MJ, Choi GS, Um SJ, Sung JM, Shin YS, Park HJ, et al. Anaphylaxis; 10 years' experience at a university hospital in Suwon. Korean J Asthma Allergy Clin Immunol. 2008; 28:298–304.
30. Somech R, Weber EA, Lavi S. Evaluation of immediate allergic reactions to cephalosporins in non-penicillin-allergic patients. Int Arch Allergy Immunol. 2009; 150:205–209.

31. Yoon SY, Park SY, Kim S, Lee T, Lee YS, Kwon HS, et al. Validation of the cephalosporin intradermal skin test for predicting immediate hypersensitivity: a prospective study with drug challenge. Allergy. 2013; 68:938–944.

32. Atanasković-Marković M, Gavrović-Jankulović M, Cirković Velicković T, Vucković O, Todorić D. Type-I hypersensitivity to ceftriaxone and cross-reactivity with cefalexin and ampicillin. Allergy. 2003; 58:537–538.

33. Pham NH, Baldo BA. beta-Lactam drug allergens: fine structural recognition patterns of cephalosporin-reactive IgE antibodies. J Mol Recognit. 1996; 9:287–296.

34. Jiao D, Liu Y, Lu X, Pan Q, Zheng J, Liu B, et al. Characteristics of anaphylaxis-inducing IgG immune complexes triggering murine passive systemic anaphylaxis. Allergy. 2013; 68:236–245.

35. Dash CH. Penicillin allergy and the cephalosporins. J Antimicrob Chemother. 1975; 1:3 Suppl. 107–118.

36. Saxon A, Beall GN, Rohr AS, Adelman DC. Immediate hypersensitivity reactions to beta-lactam antibiotics. Ann Intern Med. 1987; 107:204–215.

37. Petz LD. Immunologic cross-reactivity between penicillins and cephalosporins: a review. J Infect Dis. 1978; 137:Suppl. S74–S79.

38. Petz LD. Immunologic reactions of humans to cephalosporins. Postgrad Med J. 1971; 47:Suppl. 64–69.
40. Romano A, Guéant-Rodriguez RM, Viola M, Pettinato R, Guéant JL. Cross-reactivity and tolerability of cephalosporins in patients with immediate hypersensitivity to penicillins. Ann Intern Med. 2004; 141:16–22.

41. Macy E, Mangat R, Burchette RJ. Penicillin skin testing in advance of need: multiyear follow-up in 568 test result-negative subjects exposed to oral penicillins. J Allergy Clin Immunol. 2003; 111:1111–1115.

42. Antúnez C, Fernández T, Blanca-Lopez N, Torres MJ, Mayorga C, Canto G, et al. IgE antibodies to betalactams: relationship between the triggering hapten and the specificity of the immune response. Allergy. 2006; 61:940–946.

43. Audicana M, Bernaola G, Urrutia I, Echechipia S, Gastaminza G, Muñoz D, et al. Allergic reactions to betalactams: studies in a group of patients allergic to penicillin and evaluation of cross-reactivity with cephalosporin. Allergy. 1994; 49:108–113.

44. Blanca M, Fernandez J, Miranda A, Terrados S, Torres MJ, Vega JM, et al. Cross-reactivity between penicillins and cephalosporins: clinical and immunologic studies. J Allergy Clin Immunol. 1989; 83(2 Pt 1):381–385.

45. Katsutani N, Shionoya H. Immunogenicity of various beta-lactam antibiotic-protein conjugates and cross-reactivity of the antibodies produced in guinea pig. Int Arch Allergy Immunol. 1993; 100:128–134.

46. Miranda A, Blanca M, Vega JM, Moreno F, Carmona MJ, García JJ, et al. Cross-reactivity between a penicillin and a cephalosporin with the same side chain. J Allergy Clin Immunol. 1996; 98:671–677.

47. Sastre J, Quijano LD, Novalbos A, Hernandez G, Cuesta J, de las Heras M, et al. Clinical cross-reactivity between amoxicillin and cephadroxil in patients allergic to amoxicillin and with good tolerance of penicillin. Allergy. 1996; 51:383–386.

48. Jönsson F, Mancardi DA, Kita Y, Karasuyama H, Iannascoli B, Van Rooijen N, et al. Mouse and human neutrophils induce anaphylaxis. J Clin Invest. 2011; 121:1484–1496.

49. Posadas SJ, Pichler WJ. Delayed drug hypersensitivity reactions - new concepts. Clin Exp Allergy. 2007; 37:989–999.

50. Ishizaka A, Sakiyama Y, Nakanishi M, Tomizawa K, Oshika E, Kojima K, et al. The inductive effect of interleukin-4 on IgG4 and IgE synthesis in human peripheral blood lymphocytes. Clin Exp Immunol. 1990; 79:392–396.

52. Blunk JA, Schmelz M, Zeck S, Skov P, Likar R, Koppert W. Opioid-induced mast cell activation and vascular responses is not mediated by mu-opioid receptors: an in vivo microdialysis study in human skin. Anesth Analg. 2004; 98:364–370.
53. Simon RA, Schatz M, Stevenson DD, Curry N, Yamamoto F, Plow E, et al. Radiographic contrast media infusions. Measurement of histamine, complement, and fibrin split products and correlation with clinical parameters. J Allergy Clin Immunol. 1979; 63:281–288.
54. Ring J, Simon RA, Arroyave CM. Increased in vitro histamine release by radiographic contrast media in patients with history of incompatibility. Clin Exp Immunol. 1978; 34:302–309.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download