Abstract
Purpose
The effect of different peritoneal dialysis (PD) modalities on the decline in residual renal function (RRF) is unclear due to inconsistencies among studies. In particular, the effect of automated peritoneal dialysis (APD) modalities [continuous cyclic peritoneal dialysis (CCPD) and nightly intermittent peritoneal dialysis (NIPD)] on RRF has not been examined in a large cohort.
Materials and Methods
We conducted a single-center retrospective study to investigate the association between PD modalities and decline in RRF in 142 incident PD patients [34 on CCPD, 36 on NIPD, and 72 on continuous ambulatory peritoneal dialysis (CAPD)]. RRF was measured within 2 months from PD start and at 1 year after PD initiation.
Results
The RRF at 1 year after PD initiation was 1.98±2.20 mL/min/1.73 m2 in CCPD patients and 3.63±3.67 mL/min/1.73 m2 in NIPD patients, which were moderately lower than 4.23±3.51 mL/min/1.73 m2 in CAPD patients (p=0.064). Moreover, there was no significant difference in the 1-year rate of decline of RRF between CCPD and NIPD patients, although APD patients had a faster 1-year RRF decline rate than CAPD patients (CCPD and NIPD vs. CAPD: -45.68 and -36.69 vs. 1.17%/year, p=0.045). APD was associated with a more rapid decline in RRF in patients with end-stage renal disease undergoing PD, although multivariate analysis attenuated the significance of this finding (β=-31.50; 95% CI, -63.61 to 0.62; p=0.052).
Residual renal function (RRF) in dialysis patients is clinically important because it is clearly associated with better overall health, well-being, and survival.1-5 RRF contributes not only to salt and water removal, but also the clearance of small and medium-sized molecular weight uremic toxins. Because medium-sized molecular weight uremic toxins are not readily removed by dialysis, preservation of RRF is an important issue for patients with end-stage renal disease (ESRD) to prevent uremic symptoms and signs, including pruritus, inflammation, and mineral bone disorders. Furthermore, RRF is associated with better preservation of renal endocrine and metabolic function and superior volume homeostasis.6 Therefore, determining the risk factors associated with a decline in RRF has become an important research subject.
Some previous studies have reported that RRF is better preserved in patients on continuous ambulatory peritoneal dialysis (CAPD) than on hemodialysis.7-9 Moreover, CAPD has been reported to preserve RRF better than automated peritoneal dialysis (APD),10-13 however, other studies did not find a significant difference in the rate of decline of RRF when they compared the two peritoneal dialysis (PD) modalities.14-19
The use of APD has increased substantially over the last few years, driven primarily by improvements in cyclers and patients preferring to be able to perform relatively liberal daytime activities. Within APD two modalities can be chosen: continuous cyclic peritoneal dialysis (CCPD) and nightly intermittent peritoneal dialysis (NIPD). Therefore, the influence of dialysis modality on RRF should also be considered when to decide which APD modality to adopt. However, no study has compared the effect of APD modalities on RRF. Thus, we conducted this single-center retrospective study to investigate whether there were significant differences in the 1-year rate of decline of RRF according to the PD modalities, of CCPD, NIPD, and CAPD.
We reviewed the medical records for incident PD patients who were treated in the Yonsei University Health System (YUHS) between January 2000 and March 2011. In the absence of specific clinical indications, patients were allowed to select the modality of PD, after being fully informed about each of the PD modalities. A patient's preference or need to be relatively free to perform the activities of daily living during the daytime was a major determinant for starting dialysis therapy with APD in most cases. We excluded patients who were younger than 18 years of age, patients who did not undergo serial urea kinetic studies including measurement of RRF, patients who had residual urine volume <100 mL/day, and patients who changed their PD modality during the first year of therapy. According to the study protocol, a total of 142 clinically stable patients (34 on CCPD, 36 on NIPD, and 72 on CAPD) were finally eligible.
The study protocol was approved by the Institutional Review Board (IRB) of YUHS Clinical Trial Center. However, because this study was a retrospective medical record-based study and the study subjects were de-identified, the IRB waived the need for written consent from the patients.
CAPD patients received four exchanges per day, routinely with 2.0 L dialysate. APD patients received 4 to 5 exchanges during an 8- to 10-hour night-time dwell with 2.0 L of instilled volume, using a cycler (Home Choice APD system ver. 10.4, Baxter). CCPD patients had one or two additional 2.0 L exchanges daily, whereas NIPD patients had an empty peritoneal cavity during the daytime. In the present study, all patients were prescribed to achieve the target of weekly Kt/V urea >1.7, which is the minimal target of dialysis dose for PD patients.
Demographic and clinical data at the time of PD initiation, including age, gender, comorbidities, and primary kidney disease were reviewed from medical records. The modified Charlson Comorbidity Index-a composite score of age and multiple comorbid conditions-was used to assess the burden of chronic disease.20 RRF was assessed as an average of 24-hour urine urea and creatinine clearance measured within 2 months of beginning PD and at 1 year. RRF was normalized to 1.73 m2 body surface area and expressed as mL/min/1.73 m2. Changes in RRF during the follow-up period [1-year rate of decline of RRF (%)] were calculated as follows:
According to this calculation, a higher 1-year RRF decline rate (%) means a faster decline in RRF after PD initiation. Adequacy of PD was estimated at the same time-points by measuring weekly total Kt/V for urea. The standard peritoneal equilibrium test, described by Twardowski21 was also performed at the same time to estimate peritoneal transport characteristics and all subjects were categorized into four groups according to the value of 4-hour dialysate-to-plasma creatinine ratio (4-h D/P cr) as follows: high, ≥0.81; high average, 0.65 to 0.80; low average, 0.50 to 0.64; low <0.50. Laboratory tests results and the incidence of peritonitis were also collected.
Statistical analyses were performed using SPSS for windows version 19.0 (SPSS Inc., Chicago, IL, USA). Continuous variables are expressed as means±standard deviations and categorical variables as numbers with percentages. Baseline characteristics of the groups were compared using one-way ANOVA for continuous variables and the χ2 test for categorical variables. Pearson's correlation analysis was performed to elucidate the relationships between the rate of decline in RRF and other variables. In addition, risk factors assumed to be associated with a rapid rate of decline of RRF were evaluated using multivariate analysis. A p-value of <0.05 was considered statistically significant.
The baseline demographic, clinical, and biochemical characteristics of the patients are shown in Table 1. Age, gender, body mass index (BMI), mean arterial pressure, and biochemical parameters were similar among the three groups. Compared with patients on CAPD and CCPD, patients on NIPD tended to have higher urine volume at the time of PD initiation, although this difference did not reach statistical significance. In patients with primary kidney disease, a higher proportion of patients in the CAPD group had diabetes, although the difference was not statistically significant. Time-average HbA1c levels of diabetic patients during follow-up period were also comparable among the three groups (CCPD vs. NIPD vs. CAPD: 7.3±0.2 vs. 7.1±0.6 vs. 7.3±0.7%, p=0.717). There were no significant differences in baseline RRF, weekly Kt/V urea, 4-h D/P cr, and biochemical parameters among the three groups. Moreover, the incidence of peritonitis was not significantly different among the three groups during the follow-up period.
Fig. 1 shows the changes in RRF from baseline to 1 year after initiation of dialysis according to PD modality. The RRF at 1 year after PD initiation was 1.98±2.20 mL/min/1.73 m2 in CCPD patients and 3.63±3.67 mL/min/1.73 m2 in NIPD patients; these values were moderately lower than the average value of 4.23±3.51 mL/min/1.73 m2 in CAPD patients (p=0.064). There was no significant difference in the rate of decline of RRF between the two APD groups, but the APD groups displayed a faster 1-year rate of decline of RRF than the CAPD group (CCPD and NIPD vs. CAPD: -45.68 and -36.69 vs. 1.17%/year, p=0.045 in Fig. 2). However, there was no significant difference in the 1-year decline rate of urine volume among the three groups (CCPD vs. NIPD vs. CAPD: -20.03 vs. 16.14 vs. -30.91%/year, p=0.195 in Fig. 3). Patients who lost all of their RRF during follow-up period were 6 (17.6%) in CCPD patients, 3 (8.3%) in NIPD patients, and 4 (5.6%) in CAPD patients (p=0.325). On the contrary, patients who showed any increase in their RRF were 4 (11.8%) in CCPD patients, 7 (19.4%) in NIPD patients, and 29 (40.3%) in CAPD patients (p=0.004).
The rate of decline of RRF was negatively correlated with APD modality (APD vs. CAPD; r=-0.286, p=0.014) and baseline urine volume (r=-0.244, p=0.036) in univariate analysis. When patients were divided into two groups according to the median value of baseline RRF (3.6 mL/min/1.73 m2), there was no significant difference in the rate of decline of RRF between the two groups (>3.6 mL/min/1.73 m2 vs. ≤3.6 mL/min/1.73 m2: -14.64 vs. -28.60%/year, p=0.435). Moreover, the rates of decline of RRF were not significantly different in patients with diabetes compared with those without diabetes (DM vs. non-DM: -15.57 vs. -21.31%/year, p=0.210). On multivariate linear regression analysis using the known risk factors of rapid decline of RRF, including age, gender, DM, BMI, albumin, peritonitis episode, baseline RRF, baseline urine volume, and APD vs. CAPD, we found that the choice of APD (vs. CAPD) (β=-31.50; 95% CI, -63.61 to 0.62; p=0.052) and baseline urine volume (β=-0.51; 95% CI, -1.06 to 0.05; p=0.061) were marginally associated with a rapid rate of decline of RRF (Table 2).
RRF plays a major role in maintaining water and electrolyte balance, in maintaining erythropoietin synthesis and converting vitamin D to its active form, and in eliminating so-called middle molecules.2,4,5 RRF has therefore been recognized as a significant predictor of morbidity and mortality in patients with ESRD on dialysis,1-6 and it is important to preserve RRF as long as possible after the initiation of dialysis. To date, several risk factors, such as male gender, diabetes, uncontrolled hypertension, and heavy proteinuria, have been demonstrated to be associated with a rapid decline in RRF.7,17,19,22 Despite the importance of preserving RRF, the influence of PD modalities on RRF after the initiation of PD therapy remains unclear because of inconsistencies among the studies that may have been arisen due to the diverse experimental designs and different study populations. In particular, little is known about the effect of the APD modalities of CCPD and NIPD on the decline in RRF.
In the present study, we enrolled clinically stable incident patients in order to analyze the inherent influence of each PD modality on RRF, and found that RRF declined significantly faster in incident APD (CCPD and NIPD) patients than CAPD patients during the first year after the initiation of PD and that APD modality was associated with a more rapid decline in RRF in patients with ESRD undergoing PD, although multivariate analysis attenuated the significance of this finding. These results are consistent with those of several previous studies.10-13 Hiroshige, et al.10 assorted patients into three groups according to PD modality (CCPD, NIPD, and CAPD) and analyzed the rate of decline of RRF in each group. Recently, Michels, et al.12 stated that the risk of losing all RRF was higher in patients starting dialysis on APD than those starting on CAPD, especially in the first year. Although the results of these previous studies were similar to ours, Hiroshige, et al.10 included only a small number of patients (5 on CCPD, 8 on NIPD, and 5 on CAPD), and CCPD and NIPD groups were not compared in the latter study. We compared the rate of decline of RRF between two APD modalities in a relatively large number of patients, and confirmed that there was no significant difference in the 1-year rate of decline of RRF between the two APD modalities. After 1-year follow-up, 40 (28.2%) patients presented an improvement of RRF (4 in CCPD, 7 in NIPD, and 29 in CAPD group). Among these, 6 patients showed more than 2-fold increase in RRF. Although partial recovery of RRF in dialysis patients was reported in previous studies,23-25 the underlying mechanism remains unclear.
It is not yet completely understood how APD detrimentally affects RRF. Previous studies have shown that changes in body fluid status are related to a rapid decline of RRF in dialysis patients.26-30 In APD, most ultrafiltration occurs during an 8-hour period at night, a relatively short-time period, compared to CAPD. The intermittent nature of the APD modality may result in greater variations in hemodynamic status and possibly cause ischemia, which could exacerbate the decline of RRF. Similarly, Hufnagel, et al.11 suggested that the accelerated decline in RRF in the APD group was due to acute changes in osmotic loading and volume removal. In the present study, however, we were not able to confirm this hypothesis, although we expected that the more intermittent nature of NIPD might result in a greater decline in RRF. Our results might have been affected by the possible process of information censoring. Because patients who changed their initial dialysis modality within the first year were excluded from the analysis, it is possible that if the initial dialysis modality caused a rapid decline in RRF, early dropout could have occurred, resulting in selection bias. Therefore, further prospective studies are needed to confirm our findings.
A few observational studies reported that a higher RRF at the time of PD initiation was associated with more rapid decline in RRF during the follow-up period, although the patients with a higher RRF took a longer time to develop complete anuria.9,17,18 In our study, we did not find a relationship between higher RRF at the time of PD initiation and a rapid decline in RRF. Actually, we divided patients into two groups according to the median value of baseline RRF (>3.6 mL/min/1.73 m2 vs. ≤3.6 mL/min/1.73 m2) and compared the rate of decline of RRF. There was no significant difference in the rate of decline of RRF between the two groups. When further subgroup analysis was performed in each PD modality group, the results remained unaltered (data not shown). However, we did find that RRF declined more rapidly in patients with a high baseline urine volume, although the significance of this finding was attenuated after adjusting for other variables. The correlation between baseline urine volume and a decline of RRF may partially be explained by the choice of APD modality, which is preferred by patients who have sufficient urine volume and RRF. However, after adjustment for other variables, a moderate association remained, suggesting that baseline urine volume may in fact determine the rate of decline of RRF. It can be surmised that patients with low urine volume at the beginning of PD therapy are unlikely to experience as much of a decrease in RRF as patients with a high urine volume.
APD has been suggested to have advantage over CAPD with respect to the incidence of peritonitis, mainly due to the low number of connections required during the daytime.31-34 The result of our study did not demonstrate the superiority of APD to CAPD in reducing episodes of peritonitis, which might be due to our relatively short follow-up period.
Our present study has several limitations. First, diabetes mellitus, as the primary kidney disease, was more predominant in the CAPD group than the other groups, although this difference was not statistically significant. In the literature, diabetes has been shown to have a negative influence on the preservation of RRF in ESRD patients.7,17,22 Therefore, the rate of decline of RRF in diabetic patients might be different from that of patients with other causes of ESRD. However, when subgroup analysis was performed according to the presence of diabetes mellitus, there were similar trends in the 1-year rate of decline of RRF among the three groups (DM group, CCPD vs. NIPD vs. CAPD: -39.57 vs. -11.49 vs. 15.92%/year, p=0.057; non-DM group, CCPD vs. NIPD vs. CAPD: -51.71 vs. -58.76 vs. -11.54%/year, p=0.012). Second, the use of biocompatible PD solution was not examined. In recent years, it has been suggested that biocompatible PD solutions can result in better preservation of RRF.35-39 However, conflicting results have also been reported, and the clinical benefits of biocompatible PD solutions have not yet been fully established.40,41 Third, the follow-up duration of our study was 12 months, which is relatively shorter than other studies.4,12,42 Given that PD patients display diverse patterns of decline in RRF, a longer period of observation is required to validate our findings. Finally, the retrospective observational study design is critical limitation. However, because the choice of PD modality is based primarily on the patient's preference, randomization for dialysis is very difficult, and this limitation is unlikely to be resolved completely in studies of PD patients. Despite all inherent drawbacks, our single-center study included more CCPD and NIPD patients than previous studies, giving us more statistical power.
In conclusion, we demonstrated that RRF declined more rapidly in patients who started dialysis on APD (CCPD and NIPD), than those who started dialysis on CAPD, during the first year after the initiation of PD. Moreover, we found that there was no significant difference in the 1-year rate of decline of RRF between the two APD modalities. Therefore, we recommend that physicians should carefully monitor RRF in patients on APD, so that incremental adjustments can be made to the dialysis prescription to compensate for gradual or abrupt decreases in RRF. Furthermore, APD should be individualized according to each patient's needs and concerns. The present study was limited by its retrospective design and single-center nature. A larger prospective study with a longer follow-up time is warranted to clarify the influence of PD modality on the decline in RRF.
Figures and Tables
Fig. 1
Decline of residual renal function in CCPD patients (A), NIPD patients (B), and CAPD patients (C) from baseline to 1-year after PD initiation. CCPD, continuous cyclic peritoneal dialysis; NIPD, nightly intermittent peritoneal dialysis; CAPD, continuous ambulatory peritoneal dialysis; PD, peritoneal dialysis.

Fig. 2
Comparison of the 1-year rate of decline of residual renal function among the three groups.*vs. CAPD group, p<0.05. CCPD, continuous cyclic peritoneal dialysis; NIPD, nightly intermittent peritoneal dialysis; CAPD, continuous ambulatory peritoneal dialysis.

Fig. 3
Comparison of the 1-year rate of decline of urine volume among the three groups. CCPD, continuous cyclic peritoneal dialysis; NIPD, nightly intermittent peritoneal dialysis; CAPD, continuous ambulatory peritoneal dialysis.

Table 1
Baseline Demographic, Clinical, and Biochemical Characteristics at the Time of PD Initiation

PD, peritoneal dialysis; CAPD, continuous ambulatory peritoneal dialysis; CCPD, continuous cyclic peritoneal dialysis; NIPD, nightly intermittent peritoneal dialysis; LVH on ECG, left ventricular hypertrophy on electrocardiogram; RAS, renin-angiotensin system.
Data are presented as means±SDs or as numbers (percentages).
*Cardiovascular comorbidities were defined as coronary artery disease, cerebrovascular disease, peripheral artery disease, and congestive heart failure.
†Median value.
ACKNOWLEDGEMENTS
This work was supported by the Brain Korea 21 Project for Medical Science, Yonsei University, by a National Research Foundation of Korea grant funded by the Korean government (MEST No. 2011-0030711), and by grant A102065 from the Korea Healthcare Technology R&D Project, Ministry of Health and Welfare, Republic of Korea.
References
1. Liao CT, Chen YM, Shiao CC, Hu FC, Huang JW, Kao TW, et al. Rate of decline of residual renal function is associated with all-cause mortality and technique failure in patients on long-term peritoneal dialysis. Nephrol Dial Transplant. 2009; 24:2909–2914.

2. Marrón B, Remón C, Pérez-Fontán M, Quirós P, Ortíz A. Benefits of preserving residual renal function in peritoneal dialysis. Kidney Int Suppl. 2008; S42–S51.

3. Canada-USA (CANUSA) Peritoneal Dialysis Study Group. Adequacy of dialysis and nutrition in continuous peritoneal dialysis: association with clinical outcomes. J Am Soc Nephrol. 1996; 7:198–207.
4. Bargman JM, Golper TA. The importance of residual renal function for patients on dialysis. Nephrol Dial Transplant. 2005; 20:671–673.

5. Wang AY, Lai KN. The importance of residual renal function in dialysis patients. Kidney Int. 2006; 69:1726–1732.

6. Chandna SM, Farrington K. Residual renal function: considerations on its importance and preservation in dialysis patients. Semin Dial. 2004; 17:196–201.

7. Jansen MA, Hart AA, Korevaar JC, Dekker FW, Boeschoten EW, Krediet RT, et al. Predictors of the rate of decline of residual renal function in incident dialysis patients. Kidney Int. 2002; 62:1046–1053.

8. Lameire NH. The impact of residual renal function on the adequacy of peritoneal dialysis. Nephron. 1997; 77:13–28.

9. Moist LM, Port FK, Orzol SM, Young EW, Ostbye T, Wolfe RA, et al. Predictors of loss of residual renal function among new dialysis patients. J Am Soc Nephrol. 2000; 11:556–564.

10. Hiroshige K, Yuu K, Soejima M, Takasugi M, Kuroiwa A. Rapid decline of residual renal function in patients on automated peritoneal dialysis. Perit Dial Int. 1996; 16:307–315.

11. Hufnagel G, Michel C, Queffeulou G, Skhiri H, Damieri H, Mignon F. The influence of automated peritoneal dialysis on the decrease in residual renal function. Nephrol Dial Transplant. 1999; 14:1224–1228.

12. Michels WM, Verduijn M, Grootendorst DC, le Cessie S, Boeschoten EW, Dekker FW, et al. Decline in residual renal function in automated compared with continuous ambulatory peritoneal dialysis. Clin J Am Soc Nephrol. 2011; 6:537–542.

13. Rodriguez-Carmona A, Pérez-Fontán M, Garca-Naveiro R, Villaverde P, Peteiro J. Compared time profiles of ultrafiltration, sodium removal, and renal function in incident CAPD and automated peritoneal dialysis patients. Am J Kidney Dis. 2004; 44:132–145.

14. Bro S, Bjorner JB, Tofte-Jensen P, Klem S, Almtoft B, Danielsen H, et al. A prospective, randomized multicenter study comparing APD and CAPD treatment. Perit Dial Int. 1999; 19:526–533.

15. de Fijter CW, Oe LP, Nauta JJ, van der Meulen J, Verbrugh HA, Verhoef J, et al. Clinical efficacy and morbidity associated with continuous cyclic compared with continuous ambulatory peritoneal dialysis. Ann Intern Med. 1994; 120:264–271.

16. Hamada C, Osada S, Inoue S, Tanaka A, Fukui M, Kubota M, et al. Effects of automated peritoneal dialysis on residual urinary volume. Perit Dial Int. 2000; 20:239–241.

17. Johnson DW, Mudge DW, Sturtevant JM, Hawley CM, Campbell SB, Isbel NM, et al. Predictors of decline of residual renal function in new peritoneal dialysis patients. Perit Dial Int. 2003; 23:276–283.

18. Liao CT, Shiao CC, Huang JW, Hung KY, Chuang HF, Chen YM, et al. Predictors of faster decline of residual renal function in Taiwanese peritoneal dialysis patients. Perit Dial Int. 2008; 28:Suppl 3. S191–S195.

19. Singhal MK, Bhaskaran S, Vidgen E, Bargman JM, Vas SI, Oreopoulos DG. Rate of decline of residual renal function in patients on continuous peritoneal dialysis and factors affecting it. Perit Dial Int. 2000; 20:429–438.

20. Beddhu S, Bruns FJ, Saul M, Seddon P, Zeidel ML. A simple comorbidity scale predicts clinical outcomes and costs in dialysis patients. Am J Med. 2000; 108:609–613.

21. Twardowski ZJ. Clinical value of standardized equilibration tests in CAPD patients. Blood Purif. 1989; 7:95–108.

22. Perl J, Bargman JM. The importance of residual kidney function for patients on dialysis: a critical review. Am J Kidney Dis. 2009; 53:1068–1081.

23. Rottembourg J. Residual renal function and recovery of renal function in patients treated by CAPD. Kidney Int Suppl. 1993; 40:S106–S110.
24. Agraharkar M, Nair V, Patlovany M. Recovery of renal function in dialysis patients. BMC Nephrol. 2003; 4:9.

25. Szeto CC, Chow KM. Residual renal function and recovery of renal function. Perit Dial Int. 2007; 27:159–161.

26. Davies SJ. Preserving residual renal function in peritoneal dialysis: volume or biocompatibility? Nephrol Dial Transplant. 2009; 24:2620–2622.

27. Davies SJ, Garcia Lopez E, Woodrow G, Donovan K, Plum J, Williams P, et al. Longitudinal relationships between fluid status, inflammation, urine volume and plasma metabolites of icodextrin in patients randomized to glucose or icodextrin for the long exchange. Nephrol Dial Transplant. 2008; 23:2982–2988.

28. Davies SJ, Woodrow G, Donovan K, Plum J, Williams P, Johans AC, et al. Icodextrin improves the fluid status of peritoneal dialysis patients: results of a double-blind randomized controlled trial. J Am Soc Nephrol. 2003; 14:2338–2344.

29. Konings CJ, Kooman JP, Gladziwa U, van der Sande FM, Leunissen KM. A decline in residual glomerular filtration during the use of icodextrin may be due to underhydration. Kidney Int. 2005; 67:1190–1191.

30. Konings CJ, Kooman JP, Schonck M, Gladziwa U, Wirtz J, van den Wall Bake AW, et al. Effect of icodextrin on volume status, blood pressure and echocardiographic parameters: a randomized study. Kidney Int. 2003; 63:1556–1563.

31. Brunkhorst R, Wrenger E, Krautzig S, Ehlerding G, Mahiout A, Koch KM. Clinical experience with home automated peritoneal dialysis. Kidney Int Suppl. 1994; 48:S25–S30.
32. Holley JL, Bernardini J, Piraino B. Continuous cycling peritoneal dialysis is associated with lower rates of catheter infections than continuous ambulatory peritoneal dialysis. Am J Kidney Dis. 1990; 16:133–136.

33. Korbet SM, Vonesh EF, Firanek CA. Peritonitis in an urban peritoneal dialysis program: an analysis of infecting pathogens. Am J Kidney Dis. 1995; 26:47–53.

34. Rodríguez-Carmona A, Pérez Fontán M, García Falcón T, Fernández Rivera C, Valdés F. A comparative analysis on the incidence of peritonitis and exit-site infection in CAPD and automated peritoneal dialysis. Perit Dial Int. 1999; 19:253–258.

35. Adachi Y, Nakagawa Y, Nishio A. Icodextrin preserves residual renal function in patients treated with automated peritoneal dialysis. Perit Dial Int. 2006; 26:405–407.

36. Haag-Weber M, Krämer R, Haake R, Islam MS, Prischl F, Haug U, et al. Low-GDP fluid (Gambrosol trio) attenuates decline of residual renal function in PD patients: a prospective randomized study. Nephrol Dial Transplant. 2010; 25:2288–2296.

37. Kim S, Oh J, Kim S, Chung W, Ahn C, Kim SG, et al. Benefits of biocompatible PD fluid for preservation of residual renal function in incident CAPD patients: a 1-year study. Nephrol Dial Transplant. 2009; 24:2899–2908.

38. Rodríguez-Carmona A, Pérez Fontán M, García López E, García Falcón T, Díaz Cambre H. Use of icodextrin during nocturnal automated peritoneal dialysis allows sustained ultrafiltration while reducing the peritoneal glucose load: a randomized crossover study. Perit Dial Int. 2007; 27:260–266.

39. Williams JD, Topley N, Craig KJ, Mackenzie RK, Pischetsrieder M, Lage C, et al. The Euro-Balance Trial: the effect of a new biocompatible peritoneal dialysis fluid (balance) on the peritoneal membrane. Kidney Int. 2004; 66:408–418.

40. Fan SL, Pile T, Punzalan S, Raftery MJ, Yaqoob MM. Randomized controlled study of biocompatible peritoneal dialysis solutions: effect on residual renal function. Kidney Int. 2008; 73:200–206.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


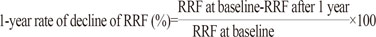

 XML Download
XML Download