Abstract
Purpose
This study used ultrasonography (US) to investigate the architectural changes in gastrocnemius muscles (GCM) after botulinum toxin injection (BoNT-A) in children with cerebral palsy (CP).
Materials and Methods
Thirteen children with CP who received a BoNT-A injection into their GCM to treat equinus were recruited (9 males and 4 females). Architectural changes in both the medial and lateral heads of the GCM from a total of 20 legs were assessed using B-mode, real-time US. Muscle thickness (MT), fascicle length (FL), and fascicle angle (FA) were measured over the middle of the muscle belly in both a resting and neutral ankle position. Measures at 1 and 3 months after the injection were compared with baseline data taken before the injection.
Results
The mean age of the subjects was 5.8 (±1.6) years. Spasticity was significantly reduced when measured by both the modified Tardieu scale and the modified Ashworth scale at 1 and 3 months after injection (p<0.05). The MT and FA of both the medial and lateral heads of the GCM were significantly reduced for both neutral and resting ankle positions at 1 and 3 months after the injection. The FL of both the medial and lateral heads of the GCM were significantly increased in a resting position (p<0.05), but not in a neutral position.
Cerebral palsy (CP) is a group of permanent motor disorders that are attributed to a non-progressive lesion in immature brains. Spasticity is the most common type of CP, and can involve disabling features that lead to limited motor learning and locomotor abilities, which can then contribute to development of joint contractures and deformities causing further functional impairments. As well, spasticity can also lead to significant structural alterations in muscles.1 Therefore, many therapeutic interventions, including physical modalities, anti-spastic drugs and orthoses, have been employed in an attempt to reduce spasticity.2 Injection of botulinum toxin type A (BoNT-A) into spastic muscles is widely used as a safe and effective intervention for pediatric spastic management. Dynamic equinus deformity is frequently targeted for BoNT-A injection, and significant beneficial effects of the injection have been repetitively reported in the literature on both spasticity reduction and functional improvement.
Notwithstanding, previous studies have revealed that toxin injection into non-spastic muscles induces muscle atrophy in both animal3,4,5,6 and human models.7 A previous report demonstrated muscle atrophy and loss of contractile tissue with repetitive toxin injection into non-spastic muscles of rabbits.8 There is a widespread concern that BoNT-A injections may compromise muscle growth in volume and exacerbate loss of underlying muscle strength, especially in young children with spastic CP. However, the impact of the toxin on spastic muscle function has been sparsely reported, especially in children with CP.
Musculoskeletal ultrasonography (US) is considered a potential tool for quantifying muscle architectural changes in vivo, revealing the arrangement of muscle fibers within a muscle.9 With the use of musculoskeletal US, morphological changes in the architecture of paretic muscles has recently been reported in children with spastic CP in comparison to both the muscles of non paretic limbs of children with CP and typically developing children.10 To the best of our knowledge, architectural changes in spastic muscles after BoNT-A injection have not yet been reported in children with CP. Therefore, the purpose of our study was to investigate the effect of BoNT-A injections on muscle architecture using US in children with spastic CP.11,12
Among children with spastic CP who visited our clinic for therapeutic intervention from March 2011 to April 2012, the children who met the following criteria were recruited for this study: 1) presence of dynamic equinus foot due to ankle spasticity, 2) without previous history of chemodenervation, serial casting, or surgery within 6 months, and 3) children whose parents agreed to BoNT-A injection for dynamic foot deformity.
In total, 13 children (9 males and 4 females) with ages ranging from 4 to 8 years (69.7±18.6 months; mean±SD) were enrolled in this study. Six limbs from 6 children with hemiplegic CP and 14 limbs from 7 children with diplegic CP underwent BoNT-A injection into their gastrocnemius muscles (GCM). Distributions of Gross Motor Function Classification System were widely distributed from level I to IV (level I, 6; level II, 4; level III, 1; level IV, 2).
Ethical approval was granted by the Institutional Review Board and Ethics Committee of Severance Hospital (#4-2007-0066). Informed consent was obtained from parents of the children for participation in the study.
Muscle tone of the ankle plantar flexor in knee extension was assessed with the modified Ashworth scale (MAS) and modified Tardieu scale (MTS). MAS is a 6-point rating scale from 0 to 4 used to gauge muscle tone. For statistical analysis, MAS grade 1(+) was converted to 2. Likewise, MAS grades 2, 3, and 4 were converted to 3, 4, and 5, respectively
As for MTS, two levels of passive range of motion (ROM) were measured, referring to R1 and R2 angles: R2 refers to the total passive ROM of ankle dorsiflexion, while R1 refers to the point in the ROM where a catch was felt during a quick stretch of the ankle plantarflexor. The ankle joint angles for R1 and R2 were measured by manual goniometry with the "neutral-null" method (dorsiflexion angle over the neutral position was counted in positive degrees, and the neutral in negative degrees13).
All measurements were taken before injection and at 3 months after injection.
BoNT-A was delivered equally to both the medial and lateral heads of the GCM. The injections were performed under the guidance of US with the child in the prone position. The total units of the toxin per child were determined depending on the severity of spasticity and body weight. Overall, 4 units per kilogram of Botox® (Allergan, Inc., Irvine, CA, USA) or 13 units per kilogram of Dyport® (Ipsen Biopharmaceuticals, Inc., Basking Ridge, NJ, USA) were delivered for each GCM. There were no serious adverse effects of the toxin injections. All children underwent physical therapy twice per week, but no additional strengthening program or intensive treatment was given to the subjects. Also, the subjects were given an ankle foot orthosis (AFO), and wearing of AFOs was encouraged during the day time as long as possible.
None of the subjects had additional serial or short leg casting after injection.
For US, subjects were positioned prone on the examination bench with their feet hanging over the edge of the bench. Tibia length (TL) was measured from the most prominent point of the lateral malleolus to the fibular head. Points at the top 25% and 30% of TL were marked with a fiber-tip pen. US images at both of these marked points of the TL were taken for measuring muscle thickness in a resting position and a neutral ankle position in order to avoid possible changes due to measurement location.
Neural ankle angle was defined as 90° between the line of the fibula and the base of the lateral foot. The neutral ankle position while taking the images was maintained by an assistant.
US images of GCMs were taken by one trained physician using B-mode and real-time ultrasonography (Accuvix V10c system; Samsung Medison Co., Discusser & Medison Building, 1003 Daechi-dong, Gangnam-gu, Seoul, South Korea) with a scanning frequency from 5 to 12 megahertz. The US image was obtained following the recommendations of Bénard, et al.14 in order to minimize measurement error.
Muscle thickness (MT) is a measurement of the longest distance between the fascia of the GCMs in a cross sectional US image. Muscle fascicle length (FL) was defined as the straight-line distance between the upper muscular fascia and the lower muscular fascia parallel to the lines of the collagenous tissue visible on the image. This measurement was consistently made in the middle of the image where the full length of the fascicle could be visualized. The fascicle angle (FA) was defined as the angle made between the upper fascia (i.e., the line of action of the tendon) and the direction of the muscle fascicles (Fig. 1).15
All measurements were taken before injection and at 3 months after injection.
Statistical analysis was conducted using IBM SPSS statistics version 16.0.0 (IBM, New York, NY, USA). For statistical analysis, a Wilcoxon signed-rank test was used to assess differences between baseline data and the data after injection. The level of significance was set at p<0.05.
Spasticity was significantly reduced after BoNT-A injection. Both R1 and R2 measured by MTS were significantly increased, while the measurements in the MAS were significantly decreased, compared with baseline data (Fig. 2).
The FL of the medial and lateral heads of the GCMs was significantly increased at 1 and 3 months after injection, compared with the baseline data measured in the resting ankle position (p<0.05). These changes were not observed in the neutral position.
Both the FA and MT of the medial and lateral heads of the GCMs in both the resting and neutral ankle positions at both 1 and 3 months after the injection were significantly decreased when compared with baseline data (p<0.05) (Table 1).
Significant reduction in muscle volume of paretic limbs in individuals with cerebral palsy as demonstrated by magnetic resonance imaging (MRI) in comparison with both non-paretic limbs and in typically developing peers has been reported in the literature.7,16 In vivo US imaging has been successfully applied in studies of muscular geometric architecture in the human body. Compared to MRI, US imaging is less expensive and more convenient and has thus gained popularity as a tool to assess the parameters of muscle geometric architecture in vivo.
According to a previous report, MT measured using US is highly correlated with the cross sectional area measured using MRI in children with spastic cerebral palsy.17 Moreover, MT measured by US was highly correlated with voluntary muscle torque in children with CP and typically-developing children,18 and also correlated with the functional level of children with CP.19 These findings suggest that the muscle thickness measured with US in children with CP can be used as an alternative measure to quantitatively evaluate muscle strength.19 Our study revealed a significant reduction in MT measured both at 25% and 30% of the TL after toxin injection.
The locations for the MT and volume measurements using US differ in the literature, with some groups reporting measurement at two-thirds of the muscle belly length,14 others measuring at the mid portion of the muscle belly,20 and some measuring at the most bulky part of the muscle belly.11 According to a previous study, there is no significant difference in measurement of the MT between the three measurement locations (proximal, central, and distal medial GCM).21 We also discovered a similar pattern in the MT changes between two of the measurement locations.
A significant reduction in MT was also previously demonstrated at 2 months after toxin injection in hemiplegic stroke patients; MT was measured at 10 days and at 2 months after injection.11 These findings are in accordance with our findings. Since the high correlation of MT with muscle volume or torque was found in previous literature,18 the reduction in muscle thickness after injection suggests negative influences of the toxin on muscle strength and growth. On the contrary, Williams, et al.22 found that muscle strength and volume in children with CP were increased, compared with baseline data, after BoNT-A injection with natural growth and standard care.
However, the subjects in that study received standard care programs before and after injection for up to 24 weeks. The increases in strength and muscle volume were greater with addition of a strengthening program with BoNT-A injection.22 On the other hand, the children of our study received only routine physical therapy. Accordingly, these differences in the intensity and frequency of physical treatment administered to the subjects may account for the discrepancy in the changes of muscle size between these two studies. Nevertheless, the intensity of physical therapy and strengthening needed to overcome the negative influence on muscle size induced by the toxin injection remains to be studied. In many previous studies, the importance of intensive physical therapy after the injection was emphasized for enhancing the functional outcome of children with CP.22,23 On the other hand, there is consistent evidence for significant muscle volume reduction in paretic limbs of spastic CP, compared with both non paretic and normally developing children.1,20,24,25 Taken together, our results also support the notion of the importance of intensive physical therapy and strengthening programs in regards to muscle size.
Notwithstanding, the evidence is inconsistent for reduction of FL. Some reports revealed no differences in FL between children with CP and typically-developing children and between paretic and non-paretic limbs,1,12,20,25,26 whereas others demonstrated a significant reduction in FL in spastic muscle.9,10 However, there are only a few reports about FL in children with CP. Shortland, et al.'s27 report demonstrated that there were no significant differences in FL between children with diplegic CP and typically developing children. Another report demonstrated smaller FL of vastus lateralis muscles in children and adolescents with CP, compared with age matched typically developing participants.9
Our study revealed a significant decrease in FA in both resting and neutral positions, whereas FL was only increased in the resting position after the toxin injection. This similar change was also demonstrated in the hemiplegic side of stroke patients 2 months after injection when the US examination was performed in the resting ankle position with knee extension.11 According to previous studies, the FL and FA of GCM changed systematically with knee and ankle position.26,28 However, the ankle and knee positions, along with the subject's position during the US imaging, were not the same across studies.11,12,14 These sampling issues may be a reason for the discrepancy of the results from previous studies in regards to FL. From this perspective, the FL changes after injection in our study in the resting position would differ from the neutral position. Resting positions of the ankle joint may differ according to spasticity of calf muscles in children with spastic CP. However, no differences in FL for ankles in a neutral position indicated that there were no significant differences in FL in the same ankle position. These findings are in accordance with Shortland, et al.'s report. However, studies of FL in children with CP are still growing. Standardization of methodology in regards to a subject's position and ankle and knee positions for examination of muscle architectural changes is needed to compare results across the literature and also to delineate their clinical implications.
FL is proportional to the maximum excursion of the muscle and velocity of contraction, and is indicative of the number of sarcomeres in series, whereas the FA is the positive angle between muscle fibers and the aponeurosis of the muscle.9 An increase in FA indicates an increase in the muscle's capacity to produce force.9,29 Previous reports demonstrated a significant relationship between MT and FA,29 as well as a significant decrease in FA in response to disuse.30,31,32 Both muscle atrophy and decrease in FA of vastus lateralis muscles may be contributing factors to the decreased force production of the quadriceps observed in CP.9 Therefore, the decreases in FA and MT after injection in our study suggest a negative influence of the toxin on muscle force production. However, there is still very limited data on morphological and structural alterations in individuals with spastic CP after intervention for spasticity. Furthermore, the functional significance of these parameters requires further investigation in these children, along with resolution of differences in sampling issues.
In general, the effects of the toxin on tone reduction begin to appear within 2 to 3 days of injection and last an average of 3 months, with a gradual return of tone followed by gains in muscle strength.28 Thus, whether the negative effect of the toxin is reversible and how long it will last are interesting research questions. In the literature, there are some suggestions that toxin injection into spastic muscle may be helpful to muscle growth in length and thereby can prevent the development of joint contracture and deformity.33 A recent report demonstrated reduction of muscle stiffness after injection in these children upon sonoelastographic evaluation.2 From this perspective, early management of spasticity with BoNT-A injection in children with spastic CP seems to be helpful to muscle growth for length and stiffness. On the other hand, the architectural changes shown in our study cautiously suggest the possibility of a negative impact of the toxin on muscle force generation in these children. Further investigation of the exact timing for disappearance of the toxin effects or the effects of repetition of the toxin injection on muscle architecture may be worthwhile to delineate the pros and cons of the toxin injection in children with CP during growth and development.
Our study has some limitations. First, there was no control group. It remains unclear whether the changes shown in our study can be induced by placebo injection or naturally. Second, the lack of long-term follow-up is another limitation. Thus the duration of the changes observed in our study remains under question. Further studies regarding the architectural changes using musculoskeletal US long-term will be helpful for the development of an optimal range for repetitive BoNT-A injection in terms of maximizing the potential benefits of the toxin while minimizing its adverse influences on muscle function. The third limitation is the lack of control of intensity and frequency of physical therapy administered to children. The influence of exercise on muscle function is another interesting subject to be addressed in a further study.
In conclusion, our study demonstrated the architectural changes of GCM after BoNT-A injection in children with spastic CP. Decreases in MT and FA after the injection may contribute to the decreased capacity of force generation as suggested in Moreau, et al.9 Muscle architecture is a primary determinant of muscle function; thus, the changes of muscle architecture after the injection can help us to determine the influence of the toxin on muscle function. In this context, the clinical implications of our study are noteworthy. The functional significance of these parameters, the timing of disappearance of the toxin effect on muscular architecture with long-term follow-up, and changes to repetitions of toxin injection have to be investigated further in order to delineate the strengths and weakness of the toxin on muscle function in children with CP.
Figures and Tables
Fig. 1
Ultrasonographic measurements of muscle thickness (A), fascicle length (B), and fascicle angle (C). (A) Muscle thickness: the longest distance between fascia of gastrocnemius muscle in cross sectional ultrasound image. (B) Fascicle length: the length of muscle fiber between fascia in longitudinal ultrasound image. (C) Fascicle angle: the angle between muscle fiber and estimated parallel fascia line in longitudinal ultrasound image.
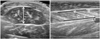
Fig. 2
Changes of spasticity after botulinum toxin type A injection. (A) Changes of modified Ashworth scale after botulinum toxin type A injection. (B) Changes of modified Tardieu scale after botulinum toxin type A injection. *Before injection-injection after 1 month are significantly different. †Before injection-injection after 3 months are significantly different: p value <0.05 in Wilcoxon signed rank test. R1, the point in the ROM where a catch was felt during a quick stretch of the ankle plantarflexor; R2, total passive ROM of ankle dorsiflexion.

References
1. Lieber RL, Steinman S, Barash IA, Chambers H. Structural and functional changes in spastic skeletal muscle. Muscle Nerve. 2004; 29:615–627.

2. Park GY, Kwon DR. Sonoelastographic evaluation of medial gastrocnemius muscles intrinsic stiffness after rehabilitation therapy with botulinum toxin a injection in spastic cerebral palsy. Arch Phys Med Rehabil. 2012; 93:2085–2089.

3. Manske SL, Boyd SK, Zernicke RF. Muscle and bone follow similar temporal patterns of recovery from muscle-induced disuse due to botulinum toxin injection. Bone. 2010; 46:24–31.

4. Shen J, Ma J, Lee C, Smith BP, Smith TL, Tan KH, et al. How muscles recover from paresis and atrophy after intramuscular injection of botulinum toxin A: study in juvenile rats. J Orthop Res. 2006; 24:1128–1135.

5. Glass GE, Hussain M, Fleming AN, Powell BW. Atrophy of the intrinsic musculature of the hands associated with the use of botulinum toxin-A injections for hyperhidrosis: a case report and review of the literature. J Plast Reconstr Aesthet Surg. 2009; 62:e274–e276.

6. Choi WH, Song CW, Kim YB, Ha CS, Yang GH, Woo HD, et al. Skeletal muscle atrophy induced by intramuscular repeated dose of botulinum toxin type A in rats. Drug Chem Toxicol. 2007; 30:217–227.

7. Schroeder AS, Ertl-Wagner B, Britsch S, Schröder JM, Nikolin S, Weis J, et al. Muscle biopsy substantiates long-term MRI alterations one year after a single dose of botulinum toxin injected into the lateral gastrocnemius muscle of healthy volunteers. Mov Disord. 2009; 24:1494–1503.

8. Fortuna R, Vaz MA, Youssef AR, Longino D, Herzog W. Changes in contractile properties of muscles receiving repeat injections of botulinum toxin (Botox). J Biomech. 2011; 44:39–44.

9. Moreau NG, Teefey SA, Damiano DL. In vivo muscle architecture and size of the rectus femoris and vastus lateralis in children and adolescents with cerebral palsy. Dev Med Child Neurol. 2009; 51:800–806.

10. Mohagheghi AA, Khan T, Meadows TH, Giannikas K, Baltzopoulos V, Maganaris CN. In vivo gastrocnemius muscle fascicle length in children with and without diplegic cerebral palsy. Dev Med Child Neurol. 2008; 50:44–50.

11. Tok F, Ozçakar L, Safaz I, Alaca R. Effects of botulinum toxin-A on the muscle architecture of stroke patients: the first ultrasonographic study. J Rehabil Med. 2011; 43:1016–1019.

12. Barber L, Hastings-Ison T, Baker R, Barrett R, Lichtwark G. Medial gastrocnemius muscle volume and fascicle length in children aged 2 to 5 years with cerebral palsy. Dev Med Child Neurol. 2011; 53:543–548.

13. Rha DW, Kim DJ, Park ES. Effect of hinged ankle-foot orthoses on standing balance control in children with bilateral spastic cerebral palsy. Yonsei Med J. 2010; 51:746–752.

14. Bénard MR, Becher JG, Harlaar J, Huijing PA, Jaspers RT. Anatomical information is needed in ultrasound imaging of muscle to avoid potentially substantial errors in measurement of muscle geometry. Muscle Nerve. 2009; 39:652–665.

15. Lichtwark GA, Bougoulias K, Wilson AM. Muscle fascicle and series elastic element length changes along the length of the human gastrocnemius during walking and running. J Biomech. 2007; 40:157–164.

16. Riad J, Modlesky CM, Gutierrez-Farewik EM, Broström E. Are muscle volume differences related to concentric muscle work during walking in spastic hemiplegic cerebral palsy? Clin Orthop Relat Res. 2012; 470:1278–1285.

17. Bandholm T, Magnusson P, Jensen BR, Sonne-Holm S. Dorsiflexor muscle-group thickness in children with cerebral palsy: relation to cross-sectional area. NeuroRehabilitation. 2009; 24:299–306.

18. Moreau NG, Simpson KN, Teefey SA, Damiano DL. Muscle architecture predicts maximum strength and is related to activity levels in cerebral palsy. Phys Ther. 2010; 90:1619–1630.

19. Ohata K, Tsuboyama T, Ichihashi N, Minami S. Measurement of muscle thickness as quantitative muscle evaluation for adults with severe cerebral palsy. Phys Ther. 2006; 86:1231–1239.

20. Malaiya R, McNee AE, Fry NR, Eve LC, Gough M, Shortland AP. The morphology of the medial gastrocnemius in typically developing children and children with spastic hemiplegic cerebral palsy. J Electromyogr Kinesiol. 2007; 17:657–663.

21. Muramatsu T, Muraoka T, Kawakami Y, Shibayama A, Fukunaga T. In vivo determination of fascicle curvature in contracting human skeletal muscles. J Appl Physiol (1985). 2002; 92:129–134.

22. Williams SA, Elliott C, Valentine J, Gubbay A, Shipman P, Reid S. Combining strength training and botulinum neurotoxin intervention in children with cerebral palsy: the impact on muscle morphology and strength. Disabil Rehabil. 2013; 35:596–605.

23. McNee AE, Gough M, Morrissey MC, Shortland AP. Increases in muscle volume after plantarflexor strength training in children with spastic cerebral palsy. Dev Med Child Neurol. 2009; 51:429–435.

24. Elder GC, Kirk J, Stewart G, Cook K, Weir D, Marshall A, et al. Contributing factors to muscle weakness in children with cerebral palsy. Dev Med Child Neurol. 2003; 45:542–550.

25. Shortland AP, Fry NR, Eve LC, Gough M. Changes to medial gastrocnemius architecture after surgical intervention in spastic diplegia. Dev Med Child Neurol. 2004; 46:667–673.

26. Gao F, Zhao H, Gaebler-Spira D, Zhang LQ. In vivo evaluations of morphologic changes of gastrocnemius muscle fascicles and achilles tendon in children with cerebral palsy. Am J Phys Med Rehabil. 2011; 90:364–371.

27. Shortland AP, Harris CA, Gough M, Robinson RO. Architecture of the medial gastrocnemius in children with spastic diplegia. Dev Med Child Neurol. 2002; 44:158–163.

28. Fehlings D. The use of botulinum toxin in paediatric hypertonia. Paediatr Child Health. 2005; 10:379–381.
29. Kawakami Y, Abe T, Fukunaga T. Muscle-fiber pennation angles are greater in hypertrophied than in normal muscles. J Appl Physiol (1985). 1993; 74:2740–2744.

30. Seynnes OR, Maganaris CN, de Boer MD, di Prampero PE, Narici MV. Early structural adaptations to unloading in the human calf muscles. Acta Physiol (Oxf). 2008; 193:265–274.

31. de Boer MD, Maganaris CN, Seynnes OR, Rennie MJ, Narici MV. Time course of muscular, neural and tendinous adaptations to 23 day unilateral lower-limb suspension in young men. J Physiol. 2007; 583(Pt 3):1079–1091.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download