Abstract
Purpose
Opioids improve pain from knee and hip osteoarthritis (OA) and decrease the functional impairment of patients. However, there is a possibility that opioids induce analgesia and suppress the physiological pain of OA in patients, thereby inducing the progression of OA changes in these patients. The purpose of the current study was to investigate the possibility of progressive changes in OA among patients using opioids.
Materials and Methods
Two hundred knee or hip OA patients were evaluated in the current prospective, randomized, active-controlled study. Patients were randomized 1:1:1 into three parallel treatment groups: loxoprofen, tramadol/acetaminophen, and transdermal fentanyl groups. Medication was administered for 12 weeks. Pain scores and progressive OA changes on X-ray films were evaluated.
Results
Overall, pain relief was obtained by all three groups. Most patients did not show progressive OA changes; however, 3 patients in the transdermal fentanyl group showed progressive OA changes during the 12 weeks of treatment. These 3 patients used significantly higher doses than others in the transdermal fentanyl group. Additionally, the average pain score for these 3 patients was significantly lower than the average pain score for the other patients in the transdermal fentanyl group.
Knee osteoarthritis (OA) is a common and progressive joint disease. With an estimated incidence rate of 240 per 100000 person-years, it is a major public health problem in the US, and often results in early retirement and joint replacement.1
For treatment of OA, exercise and drugs are recommended. Acetaminophen, in doses of up to 4 g/day, is currently recommended for the initial treatment of mild-to-moderate pain from OA of the knee.2 A systematic review of randomized controlled trials that conducted head-to-head comparisons between acetaminophen and NSAIDs showed better efficacy of and patient preference for NSAIDs, although side effects were greater.2 Opioid therapy may be considered for patients with moderate or severe pain from OA, particularly if the pain is causing functional impairment or is reducing their quality of life.3 However, this must be individualized and carefully monitored.3 Opioids are associated with a number of common adverse effects, including constipation, nausea or vomiting, pruritus, somnolence or cognitive impairment, dry mouth, tolerance or dependence, and urinary retention.3
Opioids improve OA pain, and thereby, improve patients' quality of life. On the other hand, there is a possibility that opioids induce analgesia and suppress physiological pain from OA in patients, and as a result, induce progressive joint changes, such as Charcot neuro-osteoarthropathy joint. However, to our knowledge, this adverse event has not been previously reported.
The purpose of the current study was to investigate the possibility of progressive OA changes in patients using opioids during a relatively short period, compared with oral NSAIDs, in a prospective, randomized, active-controlled study.
Informed consent was obtained from each of the participants.
The patients who participated in this study were selected from outpatients who attended our hospital for knee or hip pain. Two hundred patients were selected from 210 knee or hip pain patients who matched the following criteria. All patients had knee or hip pain for more than one month. The major inclusion criterion was the observation of OA of the knee or hip joint on examination of an anterior-posterior X-ray image in the supine position. Patients who had a history of knee or hip surgery, infection, or rheumatoid arthritis were excluded from this study. The patients completed a self-administered questionnaire to assess socio-demographic factors and the duration of knee or hip symptoms.
All patients completed a Visual Analogue Scale evaluation of pain on movement before randomization, and after 1, 4, and 12 weeks of randomized therapy.
An anterior-posterior view X-ray examination was performed in all patients in the supine position before medication and 12 weeks after treatment. We used the Kellgren-Lawrence (KL) severity classification system, which is a validated method of classifying individual joints into one of five grades, with 0 representing normal and 4 being the most severe radiographic disease.4 Evaluation was blinded and performed by five observers. If at least three of the observers concurred, the grading score was used to define the KL grade.
We measured the distance between the surface of the medial condyle of the femur and the surface of the medial tibial plateau, as well as the distance between the surface of the lateral condyle of the femur and the surface of the lateral tibial plateau; we then averaged the measurements. We defined the average distance to the knee joint space in each knee OA patient. We measured the distance between the surface of the femoral head and the surface of the acetabular roof. We defined the distance to the hip joint space in each hip OA patient. If the distance showed more than a 50% decrease after treatment, we defined the patients as showing a progressive change in OA due to treatment.
Two hundred knee or hip OA patients were evaluated in the current randomized, prospective, parallel group, active-controlled study. The patients were randomized according to the minimization method for three groups.5 We employed sex and age as stratification factors. Patients were randomized 1:1:1 to the loxoprofen group, the tramadol/acetaminophen group, or the transdermal fentanyl group. The dose of loxoprofen sodium was 60 mg three times per day, or a total of 180 mg per day. The starting dose of tramadol/acetaminophen (tramadol 37.5 mg/acetaminophen 325 mg combination tablets) was 2 tablets per day. If this dose was not effective, it was increased to 8 tablets per day. The maximum dose was 8 tablets. The transdermal fentanyl starting dose was 12.5 µg/h. If this dose was not effective, it was increased sequentially to 25, 37.5, and 50 µg/h. The maximum dose was 50 µg/h. Study medications were administered for 12 weeks. Other drugs and injections into the knee or hip joints were not allowed in any patient.
Data were compared using a Kruskal-Wallis test to compare pain scales between the three groups; a one way ANOVA with post hoc comparisons for age, symptom duration, X-ray findings, and follow-up; and the Fisher's test for dichotomous/categorical variables. p<0.05 was considered statistically significant. Results are presented as mean±standard error of the mean unless otherwise stated.
Fig. 1 is a flow diagram of this trial. Thirty patients dropped out of this study. Table 1 shows the demographic characteristics of all patients. Two hundred patients (148 female, 52 male) with a mean age of 71.0 (±7.0) years were admitted into the study. The average duration of symptoms was 11.5 (±8.0) months. The patients suffered for at least 1 month from knee or hip pain originating from OA. The pain score was not significantly different between the three groups before treatment (p>0.05).
Table 2 shows the average dose was 180±0 mg in the loxoprofen group, 2.5±1.5 tablets in the tramadol/acetaminophen group, and 19.6±5.4 µg/h in the transdermal fentanyl group.
Table 3 shows the pain scores at 1, 4, and 12 weeks of treatment. There was a significantly lower pain score in the tramadol/acetaminophen group, compared to the loxoprofen group, at each time point (p<0.05). There was a significantly lower pain score in the transdermal fentanyl group, compared with the loxoprofen or tramadol/acetaminophen groups, at each time point (p<0.05).
Table 4 shows the evaluation of the KL grade before randomization and after 12 weeks of treatment. The average of the KL grade was not significantly different between the three groups both before and after 12 weeks of treatment (p>0.05). The average diameter of the joint space before treatment in knee or hip OA was not significantly different between the three groups (p>0.05). The average joint space after 12 weeks of treatment for knee or hip OA was not significantly different between the three groups (p>0.05). There was no significant difference between the average diameter of the joint space before and after 12 weeks of treatment in each group (p>0.05). However, in the transdermal fentanyl group, 3 patients showed more than a 50% decrease in joint space after 12 weeks of treatment (Fig. 2). The 3 patients included one with knee OA and two with hip OA. The average dose of transdermal fentanyl was 29.2±12.4 µg/h in these 3 patients; there was a significantly higher dose in the 3 patients compared with the other patients in the transdermal fentanyl group (p<0.05). The average pain score was 6.3±3.3 before treatment in the 3 patients with a higher dose of fentanyl, and the average pain score in these 3 patients was not significantly different from the pretreatment pain score (6.4±4.1) in the other patients in the transdermal fentanyl group (p>0.05). The average pain score was 0.3±0.3 after 12 weeks of treatment in the 3 patients, and the average pain score in these 3 patients was significantly lower than that in the other patients in the transdermal fentanyl group (p<0.05). The changes in the pain score and the fentanyl dose before and after medication in the 3 patients were significantly greater than those in the other patients in the transdermal fentanyl group (p<0.05).
In the current study, we showed the possibility that fentanyl induces progressive changes of OA in patients during a relatively short period, compared with oral NSAIDs or tramadol administration. This finding should be taken into consideration if we administer fentanyl to knee or hip OA patients.
The use of opioids for OA pain has been previously reported. In a randomized, double-blind, active- and placebo-controlled study, treatment with tapentadol or oxycodone was effective for the management of moderate-to-severe chronic OA-related knee and hip pain.6,7 The efficacy and safety of low-dose 7-day buprenorphine patches and prolonged-release tramadol tablets in patients with chronic moderate-to-severe OA pain of the hip and/or knee were reported for a randomized controlled study.8 The systematic review of randomized trials of long-term opioid management for OA pain illustrated fair evidence for tramadol in managing osteoarthritis pain.9 In the current study, there was a significant effect of tramadol/acetaminophen and transdermal fentanyl, compared with loxoprofen, for management of pain from knee and hip OA, and this finding was consistent with previous reports.
Concerning adverse events with opioid use, Kalso, et al.10 reported adverse events from use of opioids in treating chronic non-cancer pain, including OA. They analyzed available randomized, placebo-controlled trials using the Oxford Pain Relief Database, as well as Medline, EMBASE, and the Cochrane Library.10 Specific adverse events were reported in most studies. Constipation (41%), somnolence (29%), and nausea (32%) were most frequently reported with opioids, with vomiting (15%), dizziness (20%), and itching (15%) also reported significantly more frequently than with placebo.10 However, a progressive change in OA has not been reported.
Charcot neuro-osteoarthropathy is a devastating condition affecting most commonly the foot/ankle joint.11 Several authors have reported the existence of sensory and autonomic nerve related pain in the joint.12,13 Free nerve endings and corpuscular nerve endings are found in the knee joint capsule.12 The neuronal occurrence of autonomic transmitters and autonomic innervation of tendons, ligaments, and joint capsules have also been reported.13 If the nervous system does not work normally due to severe disease in Charcot neuro-osteoarthropathy, the joint is characterized by progressive osteolysis, which can lead to multiple fractures, joint destruction, and joint dislocation, resulting in severe deformity due to non-perception of pain. Similarly, if OA patients do not feel pain while using strong analgesics, there may be a possibility that OA progressive changes could occur in these patients. In the current study, we showed the possibility that strong opioids induce a progressive change in OA among patients during a relatively short period, compared with oral NSAIDs or tramadol administration. Indeed, the average pain score after 12 weeks of treatment in 3 patients with progressive OA in the transdermal fentanyl group was significantly lower than that in the other patients in the transdermal fentanyl group.
There are some limitations to the current study. First, we included 65-70 patients in each group. This number may not be sufficient for an analysis. Second, there is a possibility that OA progression is the natural course of the disease. However, this study was a prospective, randomized study, and the OA progression occurred during 12 weeks of treatment. We believe this progression was induced by fentanyl. Third, this study was a randomized trial; however, the power analysis was not considered. In addition, the natural courses of knee and hip OA are different. Since we could not predict the effect of opioids for OA patients and the number of patients having progressive OA, we could not evaluate the sample size. Several authors have reported the natural course of knee and hip OA. After 8.1 years of follow-up in the Framingham Osteoarthritis Study, approximately 2% of women per year had developed incident radiographic disease and 1% per year had developed symptomatic knee OA.14 A population-based study in the UK showed that 2.3% of subjects per year developed incident radiographic disease during 5.1 years of follow-up.15 In a Japanese study, 2.0% of men and 3.7% of women per year developed incident radiographic disease during 3.3 years of follow-up.16 These data suggested that the rate of incident radiographic disease is slightly higher in the Japanese population than in previous epidemiologic studies in the US and Europe. As well, progression over 8 years in a community-based sample of elderly women with radiographic findings of hip osteoarthritis was also evaluated.17 After a mean of 8.3 years of follow-up, nearly two-thirds of the women, and just over half of the hips had progressed radiographically.17 A prospective, longitudinal two-year study in 463 study patients showed joint space width decreased from 2.2 mm at baseline to 1.7 mm after two years, and radiographic progression was seen in 32% of the patients.18 These previous reports indicated that there is the possibility of a difference in progression of OA changes in knee- and hip-joint patients. Thus, there is a limitation because we evaluated hip and knee OA in the same study. Fourth, it is not clear whether the progressive OA was induced by the anesthetic effect of transdermal fentanyl or vice versa (the patients with progressive OA required a higher dose of transdermal fentanyl). We need further studies with a larger number of patients to clarify these points.
In conclusion, fentanyl may induce a progressive change in knee or hip OA during a relatively short period, compared with oral NSAIDs or tramadol administration. We should monitor for this event during the use of fentanyl to treat knee or hip OA patients.
Figures and Tables
Fig. 2
An 84-year-old woman showed knee OA before treatment (A) and progressive changes in OA (B) 12 weeks after 25 µg/h transdermal fentanyl treatment. A 55-year-old man showed hip OA before treatment (C) and progressive changes in OA (D) 12 weeks after 12.5 µg/h transdermal fentanyl treatment. A 65-year-old woman showed hip OA before treatment (E) and progressive changes in OA (F) 12 weeks after 50 µg/h transdermal fentanyl treatment. OA, osteoarthritis.
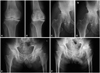
Table 3
Pain during Medication

SEM, standard error of the mean.
There was a significant difference in all scores in the tramadol/acetaminophen group, compared with the loxoprofen group (p<0.05). There was a significant difference in all scores in the transdermal fentanyl group, compared with the loxoprofen or tramadol/acetaminophen groups (p<0.05).
References
1. Centers for Disease Control and Prevention. National Center for Chronic Disease Prevention and Health Promotion. Arthritis types: overview. 2008. Available at: http://www.cdc.gov/arthritis/arthritis/osteoarthritis.htm.
2. Zhang W, Nuki G, Moskowitz RW, Abramson S, Altman RD, Arden NK, et al. OARSI recommendations for the management of hip and knee osteoarthritis: part III: changes in evidence following systematic cumulative update of research published through January 2009. Osteoarthritis Cartilage. 2010; 18:476–499.

3. Abdulla A, Adams N, Bone M, Elliott AM, Gaffin J, Jones D, et al. Guidance on the management of pain in older people. Age Ageing. 2013; 42:Suppl 1. i1–i57.
4. Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis. 1957; 16:494–502.

5. Pocock SJ, Simon R. Sequential treatment assignment with balancing for prognostic factors in the controlled clinical trial. Biometrics. 1975; 31:103–115.

6. Afilalo M, Etropolski MS, Kuperwasser B, Kelly K, Okamoto A, Van Hove I, et al. Efficacy and safety of Tapentadol extended release compared with oxycodone controlled release for the management of moderate to severe chronic pain related to osteoarthritis of the knee: a randomized, double-blind, placebo- and active-controlled phase III study. Clin Drug Investig. 2010; 30:489–505.

7. Hale M, Upmalis D, Okamoto A, Lange C, Rauschkolb C. Tolerability of tapentadol immediate release in patients with lower back pain or osteoarthritis of the hip or knee over 90 days: a randomized, double-blind study. Curr Med Res Opin. 2009; 25:1095–1104.

8. Karlsson M, Berggren AC. Efficacy and safety of low-dose transdermal buprenorphine patches (5, 10, and 20 microg/h) versus prolonged-release tramadol tablets (75, 100, 150, and 200 mg) in patients with chronic osteoarthritis pain: a 12-week, randomized, open-label, controlled, parallel-group noninferiority study. Clin Ther. 2009; 31:503–513.

9. Manchikanti L, Ailinani H, Koyyalagunta D, Datta S, Singh V, Eriator I, et al. A systematic review of randomized trials of long-term opioid management for chronic non-cancer pain. Pain Physician. 2011; 14:91–121.
10. Kalso E, Edwards JE, Moore RA, McQuay HJ. Opioids in chronic non-cancer pain: systematic review of efficacy and safety. Pain. 2004; 112:372–380.

11. Mabilleau G, Edmonds ME. Role of neuropathy on fracture healing in Charcot neuro-osteoarthropathy. J Musculoskelet Neuronal Interact. 2010; 10:84–91.
12. Schenk I, Spaethe A, Halata Z. The structure of sensory nerve endings in the knee joint capsule of the dog. Ann Anat. 1996; 178:515–521.

13. Ackermann PW, Li J, Finn A, Ahmed M, Kreicbergs A. Autonomic innervation of tendons, ligaments and joint capsules. A morphologic and quantitative study in the rat. J Orthop Res. 2001; 19:372–378.

14. Felson DT, Zhang Y, Hannan MT, Naimark A, Weissman BN, Aliabadi P, et al. The incidence and natural history of knee osteoarthritis in the elderly. The Framingham Osteoarthritis Study. Arthritis Rheum. 1995; 38:1500–1505.

15. Cooper C, Snow S, McAlindon TE, Kellingray S, Stuart B, Coggon D, et al. Risk factors for the incidence and progression of radiographic knee osteoarthritis. Arthritis Rheum. 2000; 43:995–1000.

16. Muraki S, Akune T, Oka H, Ishimoto Y, Nagata K, Yoshida M, et al. Incidence and risk factors for radiographic knee osteoarthritis and knee pain in Japanese men and women: a longitudinal population-based cohort study. Arthritis Rheum. 2012; 64:1447–1456.





 PDF
PDF ePub
ePub Citation
Citation Print
Print






 XML Download
XML Download