Abstract
Purpose
To determine the 1-year survival rate, 1-year amputation-free survival rate and the risk factors of amputation for patients with diabetic foot ulcers.
Materials and Methods
One hundred seventy-three patients with diabetic foot ulcers were included in our study. Mean patient age was 67.5 (range, 29 to 87, SD ±11.4) years. 74% of the patients were male. Time from study entry to amputation and time to death were evaluated separately as censored event times by Kaplan-Meier curves and log-rank tests. A multivariate Cox proportional hazards regression analysis was carried out for determining the risk factors of amputation.
Results
The survival rate and amputation-free survival rate were 96.5% (n=167), 65.9% (n=114), respectively, over one year study period. Severity of ulcer was the strongest significant risk factor of amputation [hazard ratio (HR): 7.99; confidence interval (CI): 3.12 to 20.47]. Peripheral artery disease was also independent risk factor of amputation (HR: 2.64; CI: 1.52 to 4.59).
A diabetic foot is a foot that exhibits any pathology that results directly from diabetes mellitus or any long-term complication of diabetes mellitus.1,2 The decisive factors for the etiology of the diabetic foot ulcers are diabetic neuropathy, macroangiopathy and the combination of neuropathy with macroangiopathy.3 Diabetic foot can present several types such neuropathic alone, mixed neuropathic-ischemic, or ischemic with infection.4,5 Furthermore, diabetic foot is one of the most common complication associated with diabetes. It is estimated that approximately from 15 to 25% of diabetes patients develop diabetic foot ulcers in the course of their disease.6 Based on recent studies, the annual incidence of diabetic foot ulcers from diabetic patients ranges from 1.0 to 4.1%.6 Diabetic foot ulcer is a frequent comorbidity of diabetes, and often results in amputation.7 Winkley, et al.8 reported that being older [hazard ratio (HR) 1.07, 95% confidence interval (CI) 1.04-1.11], and moderate ischemia (HR 2.74, 95% CI 1.46-5.14) were associated with higher mortality. Furthermore, ulcer severity was the only explanatory factor significantly associated with amputation (HR 3.18, 95% CI 1.53-6.59). Morbach, et al.9 also reported that age (HR 1.08, 95% CI 1.06-1.10), male gender (HR 1.65, 95% CI 1.18-2.32), and peripheral artery disease (PAD) (HR 1.44, 95% CI 1.05-1.98) were significant predictors for death. In addition, age (HR 1.05, 95% CI 1.01-1.10), and PAD (HR 35.34, 95% CI 4.81-259.79) were significant predictors for the first major amputation. Mortality following amputation ranges from 13 to 40% at 1 year, 35 to 65% at 3 years, and 39 to 80% at 5 years, being worse than most malignancies.6 Therefore, amputation-free survival is important in assessing the management of diabetic foot ulcers.
The purpose of this study is to determine the survival rate, amputation-free survival rate, and the risk factors of amputation for patients with diabetic foot ulcers.
This retrospective study was approved by the Institutional Review Board of our hospital. The study inclusion criteria were as follows: 1) patients with diabetic foot ulcers, who visited or referred to our clinic, a tertiary referral center, for the complex foot and ankle disease from March 2003 to October 2012; 2) patients evaluated for ankle brachial pressure index (ABPI) for ischemia. Exclusion criteria were 1) patients who did not meet the definition of diabetic foot ulcers; 2) patients who did not know whether alive or not by our medical records or telephone interview.
We searched the records for ICD-10 codes including E10.7 "diabetic foot, type 1", E11.7 "diabetic foot, type 2", E14.70 "diabetic foot with ulcer", E14.71 "diabetic foot with ulcer and gangrene", and E14.78 "diabetic foot with multiple complications". Two hundred fifty five patients met these diagnoses. After implementation of inclusion and exclusion criteria, 173 patients were enrolled in our study.
We reviewed age, gender, type of diabetes, type of treatment for diabetes, and duration of diabetes using medical record.
The literature lists smoking, peripheral artery disease, severity of ulcer, duration of ulcer, inadequate sugar control (high level of glycosylated hemoglobin), and the presence of diabetic complications (e.g., macrovascular, microvascular, nephropathy, neuropathy, and cerebrovascular) as risk factors for mortality and amputation of patients who suffered diabetic foot ulcers.4,5,6,8,9,10,11 Therefore, we reviewed such risk factors using medical record.
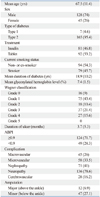
Current smoking status was divided into current smoker, ex-smoker, and non-smoker. The degree of ischemia was assessed using the ABPI. PAD was defined by an ABPI <0.9. A handheld Doppler was used to identify foot pulses (VasoGuard, Nicolet Vascular Inc., Madison, WI, USA) and measure the brachial and ankle systolic pressure together with a sphygmonometer after patient rest for 5 minutes.12 The severity of ulcer was determined using the Wagner Classification System (Table 1).11,13 We divided the severity of ulcer into 2 groups; Group 1 being the high grade of ulcer (grade 3, 4, 5) and Group 2 the low grade of ulcer (grade 0, 1, 2). The duration of ulcer was recorded from the first presentation (using medical record) to the date visited or referred to our clinic. Glycosylated hemoglobin was measured at baseline and any follow-up period. The mean percentage of glycosylated hemoglobin values was derived from baseline and any follow-up period. Macrovascular complications were defined as the presence of a history of angina pectoris or myocardial infarction, any positive cardiac stress test result, or pathologic signs on coronary angiography.8,9 Microvascular complication was defined as retinopathy (background or proliferative) measured using digital fundal examination.8 Nephropathy was defined as presented of macroalbuminuria or on dialysis.8 Assessment of protective pain sensation and sensory neuropathy was made using a Semmes-Weinstein monofilament testing.6 Neuropathy was defined as inability to perceive 10 g of force or application of a 5.07 monofilament at any sites of the forefoot (great toe and base of first, third, and fifth metatarsals).6 Cerebrovascular complications were defined as the presence of any event of neurologic deficiency, whether persistent or resolved.9
Minor amputations were defined as removal of a part of the foot distal to the transverse tarsal joint with preservation of the talus and calcaneus. Major amputations were defined as transtibial amputation.14
After all data were collected, we identified current status of survival and amputation using medical record. Patients who visited our hospital within 1 month from end of data collection period for any causes were regarded as alive patients. Forty-four patientswhose current status of survival remained, unknown, therefore, we contacted patients or their family members by telephone, and asked patient's current status.
Baseline variables were described depending on their distributions by means, standard deviations (SDs), ranges, or frequency tables. Time from the study entry to amputation and time from the study entry to death were evaluated separately as censored event times by Kaplan-Meier curves and log-rank tests. To assess the relationship between the baseline variables and incidence of death and amputation, Cox proportional hazards regression analysis was separately carried out for each variable. A multivariate Cox regression model was fitted with diabetes-associated amputations as the dependent variable and adjusted for clinical and demographic characteristics. The following factors were included in the analysis as possible predictors or confounders: gender, age, duration of diabetes, smoking status, presence of peripheral artery disease, severity of ulcer, duration of ulcer, glycosylated hemoglobin level, and diabetic complications such as macrovascular, microvascular, nephropathy, neuropathy, and cerebrovascular complication. Null hypotheses of no difference were rejected if p-values were less than 0.5. All analyses were carried out using SPSS 18.0 window version (SPSS Inc., Chicago, IL, USA).
The mean duration of follow up was 14.6 (range 1 to 84, SD±15.9) months. One hundred sixty-five (95.4%) patients were type 2 diabetes, and 80 (46.2%) patients were treated with insulin injection. The mean duration of diabetes was 18.9 (range, 0 to 42, SD±11.4) years. Fifty-nine (34.1%, n=59) patients underwent an amputation over one year study period. Twelve (6.9%) underwent major amputations (amputation above the ankle), and there were forty-seven (27.1%) minor amputations (below the ankle) (Table 2).14
The survival rate was 96.5% (n=167) for diabetic foot ulcers patients over the 1 year study period. All amputation-free survival rate was 65.9% (n=114), and major amputation-free survival rate was 93.1% (n=161) over the study period of 1 year.
Seventy-nine patients (45.7%) were active smokers, and 94 patients (54.3%) were non- or ex-smokers. The incidence of PAD over the year was 49 (28.3%). The prevalence of Grade 1 ulcer severity was 75 (43.4%), followed by Grade 3 (37, 21.4%), Grade 4 (27, 15.6%), Grade 2 (18, 10.4%), and Grade 0 (16, 9%). The mean ulcer duration was 3.7 months (range, 0-35, SD±5.24 months). The mean glycosylated hemoglobin was 7.43% (range, 4 to 13, SD±1.48%). The prevalence of neuropathy complication was 136 (78.6%), followed by nephropathy (71, 41%), retinopathy (58, 33.5%), macrovascular (45, 26%), and cerebrovascular complication (28, 16.2%). In separate univariate Cox proportional hazards regression models, severity of ulcer and PAD were significant risk increasing factor, and high glycosylated hemoglobin level was a significant preventive factor (Table 3). Multivariate Cox proportional hazards regression analysis showed that high grade of ulcer severity (Grade from 3 to 5) was the strongest significant independent predictor of amputation for diabetes patients with diabetic foot ulcers (HR: 7.99; CI: 3.12 to 20.47) (Fig. 1). Multivariate analyses also found PAD (ABI <0.9) to be a significant risk factor for amputation (HR: 2.64; CI: 1.52 to 4.59) (Fig. 2, Table 4).
The aim of this study was to determine the survival rate, amputation-free survival rate, and the risk factors for amputation for patients with diabetic foot ulcers. Our main findings demonstrated that 1-year survival rate of diabetic foot ulcers was about 97% and all amputation-free survival was about 66%. Severity of ulcer was the strongest significant risk factor of amputation for diabetes patients with diabetic foot ulcers. PAD (ABI <0.9) was also significant risk factor of amputation for diabetes patients with diabetic foot ulcers.
There is limitation to this study. First, this study was conducted on a retrospective basis. Therefore, it is somewhat difficult to determine a causal relationship between variables with amputation or mortality. Second, we could not exclude selection bias, because our hospital is a tertiary referral center for the complex foot and ankle disease. Patients who visited or were referred to our clinic might have already had worsened diabetic foot conditions. Therefore, the results of this study might not be applied to the general population.5,8 However, major amputation rate was similar or slightly lower than other earlier study.9 We decided amputation level fully consideration of patient's conditions such severity of wound, ABI level, status of sugar control, patients and their family member's concern about limb loss and so on, and confirmed multidisciplinary approach. Therefore, we performed amputations as little as possible. Third, in this study, we calculated survival rate for relatively short period. As a result, our survival rate was slightly higher than in previous studies. However, we thought that different ethnic group in previous studies was possible reason for the difference. Future study is needed with other ethnic groups. Fourth, the comparison between major and minor amputation could provide more detailed information regarding the prognosis of diabetic foot ulcers. However, the statistical analysis was not feasible due to small case number of major amputation.
The demographics of our study population were remarkably similar to other cohorts studied for diabetic foot ulcers over shorter (18 months) or longer period (≥5 years) in terms of patient age, gender, treatment of diabetes, and duration of diabetes.5,8,9 Type 2 diabetes patients were included at a slightly higher rate than other studies.5,8,9,11
The survival rate was 96.5% (n=167) for diabetic foot ulcers patients over the study period of 1 year. All amputation-free survival rate was 65.9% (n=114), and major amputation-free survival rate was 93.1% (n=161) over the study period of 1 year. Winkley, et al.8 found a survival rate of 84%, and amputation-free survival rate of 84.5% at 18 months in a population-based prospective cohort study of people with their first diabetic foot ulcer. Morbach, et al.9 observed a 1-year survival rate of 84.6%, and an amputation rate of 15.4%. However, they excluded minor amputations (below the ankle). Our study included all amputations of the lower extremities, and we found that major amputation rate was 6.9% over the study period of 1 year.
A high grade of ulcer severity (Grade from 3 to 5) markedly increased the risk of amputation in our study (HR: 7.99; CI: 3.12 to 20.47). This result was compatible with the clinical observation that a more extensive wound was associated with a more extensive surgical management, such as amputation. Sun, et al.11 showed that a high grade of Wagner classification strongly increased the risk of amputation [odds ratio (OR): 13.10; CI: 8.74 to 19.65], similar to our study.
PAD is considered to be a marker for atherosclerosis. Hoorn defined PAD as ABPI <0.9, included those with absent foot pulses, and found that it was more prevalent among those with known diabetes.12 The Strong Heart Study with a population cohort of American Indians to investigate risk factors for cardiovascular disease (CVD) found that the relative risk of CVD mortality in people with PAD was 1.69.15 Furthermore, Pscherer, et al.5 showed that PAD was the strongest significant independent predictor of amputation for diabetes patients with diabetic foot ulcers (HR: 5.13; CI: 4.27 to 6.16), similarly to our study (HR: 2.64; CI: 1.52 to 4.59).
Previous studies showed that poor diabetes control is a risk factor for limb loss in diabetic patients.5,16 Pscherer, et al.5 found that patients with a mean glycosylated hemoglobin level above 7.5% had 20% higher risk of amputation compared to patients with below 7.5%. However, Winkley, et al.8 showed that having lower glycosylated hemoglobin level was associated with higher mortality (HR: 0.73; CI: 0.56 to 0.96). Similarly, our study showed that mean glycosylated hemoglobin level below 7.5% had 52% higher risk of amputation, compared to patients with above 7.5% (HR: 0.52; CI: 0.29 to 0.92). It is quite reasonable to assume that glycosylated hemoglobin level showed opposite results in earlier studies, because glycosylated hemoglobin serves as a marker for average blood glucose levels for a few months prior to the measurement, and it does not reflect the real risk of amputation for long term complication of diabetic foot ulcers. A higher amputation rate in better long term glycemic control seems counterintuitive. A possible explanation for the result is that patients with more advanced diabetes mellitus could be exposed to better surveillance and more intensive medical treatment. However, this explanation is beyond the scope of this study and need further investigation.8 Consequently, we conclude that clinician should consider the severity of ulcer and the presence of peripheral artery disease when evaluating diabetic foot ulcers. However, recent status of blood glucose is not a significant prognostic factor.
Figures and Tables
Fig. 1
Kaplan-Meier curves for amputation-free survival of diabetes patients with diabetic foot ulcers over 1 year, depending on severity of ulcer (log-rank test p<0.01).

Fig. 2
Kaplan-Meier curves for amputation-free survival of diabetes patients with diabetic foot ulcers over 1 year, depending on mean ABPI value (log-rank test p<0.01). ABPI, ankle brachial pressure index.

References
2. Boulton AJ. [The diabetic foot]. Khirurgiia (Sofiia). 2001; 57:5–8.
3. Stiegler H. [Diabetic foot syndrome]. Herz. 2004; 29:104–115.
4. Lauterbach S, Kostev K, Kohlmann T. Prevalence of diabetic foot syndrome and its risk factors in the UK. J Wound Care. 2010; 19:333–337.

5. Pscherer S, Dippel FW, Lauterbach S, Kostev K. Amputation rate and risk factors in type 2 patients with diabetic foot syndrome under real-life conditions in Germany. Prim Care Diabetes. 2012; 6:241–246.

6. Singh N, Armstrong DG, Lipsky BA. Preventing foot ulcers in patients with diabetes. JAMA. 2005; 293:217–228.

7. Gregg EW, Sorlie P, Paulose-Ram R, Gu Q, Eberhardt MS, Wolz M, et al. Prevalence of lower-extremity disease in the US adult population >=40 years of age with and without diabetes: 1999-2000 national health and nutrition examination survey. Diabetes Care. 2004; 27:1591–1597.

8. Winkley K, Stahl D, Chalder T, Edmonds ME, Ismail K. Risk factors associated with adverse outcomes in a population-based prospective cohort study of people with their first diabetic foot ulcer. J Diabetes Complications. 2007; 21:341–349.

9. Morbach S, Furchert H, Gröblinghoff U, Hoffmeier H, Kersten K, Klauke GT, et al. Long-term prognosis of diabetic foot patients and their limbs: amputation and death over the course of a decade. Diabetes Care. 2012; 35:2021–2027.
10. Abbott CA, Carrington AL, Ashe H, Bath S, Every LC, Griffiths J, et al. The North-West Diabetes Foot Care Study: incidence of, and risk factors for, new diabetic foot ulceration in a community-based patient cohort. Diabet Med. 2002; 19:377–384.

11. Sun JH, Tsai JS, Huang CH, Lin CH, Yang HM, Chan YS, et al. Risk factors for lower extremity amputation in diabetic foot disease categorized by Wagner classification. Diabetes Res Clin Pract. 2012; 95:358–363.

12. Beks PJ, Mackaay AJ, de Neeling JN, de Vries H, Bouter LM, Heine RJ. Peripheral arterial disease in relation to glycaemic level in an elderly Caucasian population: the Hoorn study. Diabetologia. 1995; 38:86–96.

13. Sharp CS, Bessman AN, Wagner FW Jr, Garland D. Microbiology of deep tissue in diabetic gangrene. Diabetes Care. 1978; 1:289–292.

14. Wukich DK, Hobizal KB, Brooks MM. Severity of diabetic foot infection and rate of limb salvage. Foot Ankle Int. 2013; 34:351–358.





 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download