Abstract
Purpose
The function of regulatory B lymphocytes is known to be abnormal in inflammatory diseases. However, a recent study indicates that IL-10+ B cells seem to be expanded in rheumatoid arthritis (RA). Therefore, the state of IL-10+ B cells in the peripheral blood from RA patients and healthy controls were investigated.
Materials and Methods
CD19+ cells in peripheral blood mononuclear cells were purified from blood samples of RA patients and age and gender-matched healthy controls, and stimulated with CD40 ligand and CpG for 48 hours. Then, intracellular IL-10 in CD19+ cells was analyzed using flow cytometry.
Results
There was no significant difference in the proportion of IL-10+ B cells between 10 RA patients and 10 healthy controls (RA, 0.300±0.07 vs. healthy control 0.459±0.07, p=0.114). The proportion of induced IL-10+ B cells to total B cells in RA patients was significantly higher than those in controls (RA, 4.44±3.44% vs. healthy control 2.44±1.64%, p=0.033). However, the proportion of IL-10+ B cells to total B cells correlated negatively with disease activity in RA patients (r=-0.398, p=0.040). Erythrocyte sedimentation rate or C-reactive protein or medication was not associated with the proportion of IL-10+ B cells.
Rheumatoid arthritis (RA) is an autoimmune disease characterized by synovitis and other system involvement. The pathogenesis of RA has mainly been attributed to Th17 cells and its interaction with various cells including fibroblast-like synoviocytes. However, B cells have a role in RA pathogenesis by secreting antibodies such as rheumatoid factor and anti-cyclic citrullinated peptide antibody, and have been a therapeutic target, as shown by B cell depletion therapy with anti-CD20 antibody.1,2 In addition, the depletion of mature B cells in the mouse model of arthritis delayed development of arthritis.3 However, in animal models of other inflammatory diseases such as experimental autoimmune encephalomyelitis or contact hypersensitivity, B cell deficiency aggravated inflammation.4,5 In animal model of arthritis, a subset of B cells had anti-inflammatory function.6,7
The regulatory function of B cells is beginning to be understood, and several subsets of regulatory B cells have been suggested.8,9 B cell subsets with regulatory function have been designated as B-1 cell, marginal zone B cell, transitional-2 marginal zone precursor B cell or interleukin-10 (IL-10) secreting B cells.9 Although several surface markers such as CD19+CD24hiCD38hi or CD24hiCD27+ have been proposed,10,11 a definite cell surface phenotype has not yet been established. In mouse, IL-10 is produced mainly by the CD1dhiCD5+ B-cells, which share characteristics of both B-1 and marginal zone B cells,9 and also by the CD19lowCD5+ B cells in the blood of food allergy patients.12 These IL-10 secreting B cells are called as B10 cells or Br1 cells.9,13
IL-10 secreting B cells have been shown to suppress T cell proliferation and T cell-dependent inflammatory function.14 A recent functional study reported that IL-10 secreting B cells are increased in patients with RA and other inflammatory diseases, and that these cells play a central role in immune regulatory function.10 Furthermore, the function of B cells is shown to be impaired in patients with systemic lupus erythematosus,11 and the regulatory B cell population decreased during early period of inflammation in mouse model of collagen-induced arthritis.15 However, the exact roles of regulatory B cells played in autoimmune disease remain unclear.
To evaluate the status of IL-10+ B cells in RA according to the disease activity, we induced the production of IL-10+ B cells after B cell activation and analyzed the relationship between IL-10+ B cells and disease severity in RA patients.
Twenty-eight RA patients and healthy controls were enrolled. RA was diagnosed according to the 1987 American College of Rheumatology criteria or 2010 American College of Rheumatology/European League Against Rheumatism criteria.16,17 Data collected included tender joint count, swollen joint count, patient's global assessment, erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), rheumatoid factor or anti-cyclic citrullinated peptide antibody, and current medication. RA patients could be grouped by disease activity by DAS 28 calculated using CRP (DAS28-CRP): <2.6, remission; ≤3.2, low disease activity; >3.2 and ≤5.1, moderate disease activity; >5.1, high disease activity.18,19 This study protocol was reviewed and approved by the Institutional Review Board of Chungnam National University Hospital (IRB No. 1101-31), and performed according to the Declaration of Helsinki. All subjects provided their informed written consent before participation.
Peripheral blood mononuclear cells (PBMC) were isolated from heparinized blood samples of RA patients and healthy subjects by the Ficoll-density gradient methods (Ficoll-Paque™ Plus, GE Healthcare, Uppsala, Sweden). B cells were purified from PBMC by positive selection using anti-CD19 MACS Microbeads (Miltenyi Biotec, Teterow, Germany). For IL-10+ B cell induction, purified CD19+ B cells (5×105 cells) were incubated with CpG (ODN 2006, 10 µg/mL; Invivogen, San Diego, CA, USA) and CD40 ligand (CD40L, 1 µg/mL; R&D Systems, Minneapolis, MN, USA) for 48 hours in Roswell Park Memorial Institute medium 1640 with 10% fetal bovine serum in a 96-well culture plate at 37℃ as previously described.5
CD19+ cells were stimulated with phorbol 12-myristate 13-acetate (PMA; 50 ng/mL; Sigma-Aldrich, St. Louis, MO, USA) and ionomycin (250 ng/mL; Sigma-Aldrich) in the presence of monensin (Golgistop™; BD Biosciences, San Jose, CA, USA) for 5 hours immediately after purification or the last 5 hours after incubation of CD19+ cells for 2 days. Then, cells were permeabilized with BD Cytofix/Cytoperm solution (BD Biosciences) according to the manufacturer's instructions and stained with phycoerythrin-labeled anti-human IL-10 (eBioscience, San Diego, CA, USA), and flow cytometry was performed with a FACSCanto (BD, Franklin Lakes, NJ, USA) to study surface markers and intracellular cytokine levels. FlowJo (version 8.8.4, Tree Star, Ashland, OR, USA) was used to analyze flow cytometry data.
The t-test and analysis of variance were used to compare the levels of cytokine-producing B cells between groups. The correlation of clinical characteristics and IL-10+ B cells was estimated by Pearson's correlation analysis. Two-tailed null hypotheses of no difference were rejected if p values were less than 0.05. SPSS version 20 for Windows was used for all statistical tests (SPSS Inc., Chicago, IL, USA).
Among 28 RA patients, 22 were female (78.6%) and mean age (±SD) was 57.0±14.6 year-old. Median disease duration was 22 (range: 0-108) months, and rheumatoid factor was positive in 26 patients (92.9%).
Ten patients were diagnosed as RA for the first time at the time of the study. Laboratory characteristics were as follows: ESR, 52.0±38.4 mm/hr; CRP, 2.59±3.62 mg/dL; DAS28-ESR, 4.59±2.04; DAS28-CRP, 3.36±1.57. Using disease activity by DAS28-CRP, 9 RA patients were categorized into remission group, 2 low disease activity group, 12 moderate disease activity group, and 5 high disease activity group. Methotrexate was used in 10 RA patients, prednisolone in 19, or leflunomide in 2 for treatment of RA.
There was no significant difference in the proportion of IL-10+ B cells between 10 RA patients and 10 healthy controls (RA, 0.300±0.07 vs. healthy control 0.459±0.07, p=0.114). The proportion of IL-10+ B cells was not correlated with disease activity, DAS28-CRP (r=0.065, p=0.858). Thus, induction of IL-10+ B cell using CD40L and CpG was performed. There was an increase of IL-10+ B cells induced by CD40L and CpG in 18 RA patents compared with age and gender-matched 18 healthy controls (RA, 4.44±3.44% vs. healthy control 2.44±1.64%, p=0.033, by t-test) (Fig. 1). To investigate the relationship of age and IL-10+ B cell induction, we analyzed normal controls, and found no significant relationship between age and IL-10+ B cells (r=0.035, p=0.895).
During this study, preliminary data on active RA patients revealed a low proportion of induced IL-10 producing B cells. Thus, 10 more RA patients were enrolled to investigate the relationship. Among the 28 RA patients, there was negative correlation between disease activity (DAS28-CRP) and induced IL-10+ B cells (r=-0.398, p=0.040, by correlation analysis) (Fig. 2). In addition, the RA patients group had a negative correlation between age and IL-10+ B cells (r=-0.525, p=0.004, by correlation analysis), whereas age and activity in RA group was positively correlated (r=0.409, p=0.031, by correlation analysis). The correlation of IL-10+ B cells with ESR or CRP was not significant (p=0.241 and p=0.314, respectively). We investigated the difference between newly diagnosed patients (n=10) and patients with a flare-up of preexisting arthritis (moderate or high activity group, n=7), and found that the proportion of induced IL-10+ B cells was not different between groups (newly diagnosed patients, 3.10±2.41% vs. flareup patient, 2.51±2.18%, p=0.621, by t-test). Moreover, methotrexate or prednisolone use was not associated with IL-10 producing B cells (p=0.147 and p=0.325, respectively, by t-test).
In this study, we investigated the abnormality of B cells secreting IL-10. A previous study showed that the proportion of IL-10+ B cell was elevated in RA or other rheumatic diseases, however, the study did not investigate the association with clinical characteristics. The proportion of IL-10+ B cells was not different between RA patients and healthy controls. We found that disease activity was negatively associated with induction of IL-10 in B cells. The proportion of induced IL-10+ B cell was also associated with age in RA patients. However, the association of age and IL-10+ B cells in normal controls was not evident. Elderly people with RA may present with more severe manifestations than young patients.20,21 We recruited severe arthritis patients to investigate the association of activity and IL-10+ B cells. Therefore, the patient group with severe arthritis was older and it would therefore be important to investigate the association between age and disease activity in this cohort. Taking these results into consideration, the differentiation to IL-10+ B cells may be determined prior to CD40L and CpG stimulation or the interaction among various cells may be essential to induce IL-10.
Although human regulatory B cell has been elucidated, transcription factors or exact effector mechanism remain uncertain. Nevertheless, it is clear that IL-10 production is an important and unique characteristic of regulatory B cells compared to other B cell subsets. IL-10 secreting B cells can be detected by flow cytometry after PMA-ionomycin stimulation (B10 cell) or induced by stimulation of CD40 and toll-like receptor (B10pro).10 These cells can exert their regulatory function, through IL-10, on T cells, macrophages, dendritic cells or plasma cells.9 It was reported that CD19+CD24hiCD38hi B cells include the majority of IL-10+ B cells, and that IL-10 secretion in these cells is impaired in patients with systemic lupus erythematosus.11 However, we report here that the proportion of IL-10+ B cell was significantly increased in RA patients. Thus, it was not easy to understand the abnormality of IL-10+ B cell in RA. We found in this study that disease activity was negatively associated with IL-10+ B cells. Based on our results that the frequency of regulatory B cell without induction is not different, the key issue of regulatory B cells in RA may be the impaired induction of regulatory B cells. Recently, Ma, et al.22 reported reduction of regulatory B cells in patients with new-onset RA. They showed that IL-10+CD5+CD1d+ B cell or IL10+TIM-1+ B cells without induction were decreased, although our results did not show the decrease of IL-10+ B cells without induction. The study by Ma, et al. may be supported by this study. However, it should be noted that CD5, CD1d, or TIM-1 has not been established as surface markers in human regulatory B cells as opposed to mouse regulatory B cells.23,24
The frequency of IL-10+ B cells was increased in RA patients, particularly in patients with lower disease activity. However, RA has an immunologic environment to stimulate B cells in several aspects as follows; IL-10 secreting B cells are induced effectively by CD40L, and CpG. CD40L, also known as CD154, is implicated in RA pathogenesis. The enhanced expression of CD40L on T cells of RA patients was found to be responsible for the presence of highly activated B cells (CD80+CD86+), producing anti-cyclic citrullinated peptide antibodies in the synovial compartment of patients.25,26 B cell-activating factor (BAFF), which is increased in active RA, can also promote the production of IL-10 in B cells.27,28 Therefore, enhanced CD40L expression on T cells, together with increased levels of BAFF in active arthritis, comprise an inflammatory milieu in RA that favors the production of IL-10+ regulatory B. Despite this, the fact that IL-10 was increased only in inactive arthritis is intriguing. Perhaps, IL-10+ B cell induction may be impaired in active arthritis. Moreover, in an animal model of human arthritis, collagen-induced arthritis showed decreased frequencies of IL-10 producing B cells in both spleen and draining lymph nodes during the acute stage of disease, which is compatible with our present results.15 In addition, the adoptive transfer of expanded IL-10+ B cells could ameliorate disease.6,15 Therefore, the therapeutic induction or transplantation of regulatory B cell population could be an effective therapeutic strategy in acute stage or early arthritis.
In conclusion, the proportion of induced IL-10+ B cell increased in RA patients compared to healthy control and was negatively correlated with disease activity in RA.
Figures and Tables
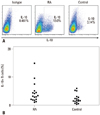 | Fig. 1The IL-10+ B cell was increased in RA patients compared to control patients. B cells from patients and controls were stimulated with CD40L and CpG for 48 hours and IL-10 intracellular staining was performed. (A) The representative plot of IL-10+ B cell in RA patients and healthy control. (B) The proportion of IL-10+ B cells among B cells was elevated in RA patients compared to normal control (p=0.033, by t-test). RA, rheumatoid arthritis; IL-10, interleukin-10. |
 | Fig. 2The correlation of IL-10+ B cell and DAS28-CRP. The proportion of IL-10+ B cells was negatively correlated with RA disease activity measured using DAS28-CRP (r=-0.398, p=0.040, by correlation analysis). DAS28-CRP, 28-joint disease activity score calculated using C-reactive protein; RA, rheumatoid arthritis; IL-10, interleukin-10. |
ACKNOWLEDGEMENTS
This work was supported by Chungnam National University Hospital Research Fund 2010 and by Basic Science Research Program-through the National Research Foundation of Korea (NRF) funded-by the Ministry of Education, Science and Technology (2012-0008388).
References
1. Nakken B, Munthe LA, Konttinen YT, Sandberg AK, Szekanecz Z, Alex P, et al. B-cells and their targeting in rheumatoid arthritis--current concepts and future perspectives. Autoimmun Rev. 2011; 11:28–34.

2. Moura RA, Graca L, Fonseca JE. To B or not to B the conductor of rheumatoid arthritis orchestra. Clin Rev Allergy Immunol. 2012; 43:281–291.

3. Yanaba K, Hamaguchi Y, Venturi GM, Steeber DA, St Clair EW, Tedder TF. B cell depletion delays collagen-induced arthritis in mice: arthritis induction requires synergy between humoral and cell-mediated immunity. J Immunol. 2007; 179:1369–1380.

4. Wolf SD, Dittel BN, Hardardottir F, Janeway CA Jr. Experimental autoimmune encephalomyelitis induction in genetically B cell-deficient mice. J Exp Med. 1996; 184:2271–2278.

5. Watanabe R, Fujimoto M, Ishiura N, Kuwano Y, Nakashima H, Yazawa N, et al. CD19 expression in B cells is important for suppression of contact hypersensitivity. Am J Pathol. 2007; 171:560–570.

6. Mauri C, Gray D, Mushtaq N, Londei M. Prevention of arthritis by interleukin 10-producing B cells. J Exp Med. 2003; 197:489–501.

7. Evans JG, Chavez-Rueda KA, Eddaoudi A, Meyer-Bahlburg A, Rawlings DJ, Ehrenstein MR, et al. Novel suppressive function of transitional 2 B cells in experimental arthritis. J Immunol. 2007; 178:7868–7878.

8. Fillatreau S, Sweenie CH, McGeachy MJ, Gray D, Anderton SM. B cells regulate autoimmunity by provision of IL-10. Nat Immunol. 2002; 3:944–950.

9. DiLillo DJ, Matsushita T, Tedder TF. B10 cells and regulatory B cells balance immune responses during inflammation, autoimmunity, and cancer. Ann N Y Acad Sci. 2010; 1183:38–57.

10. Iwata Y, Matsushita T, Horikawa M, Dilillo DJ, Yanaba K, Venturi GM, et al. Characterization of a rare IL-10-competent B-cell subset in humans that parallels mouse regulatory B10 cells. Blood. 2011; 117:530–541.

11. Blair PA, Noreña LY, Flores-Borja F, Rawlings DJ, Isenberg DA, Ehrenstein MR, et al. CD19+CD24hiCD38hi B cells exhibit regulatory capacity in healthy individuals but are functionally impaired in systemic lupus erythematosus patients. Immunity. 2010; 32:129–140.

12. Lee JH, Noh J, Noh G, Choi WS, Lee SS. IL-10 is predominantly produced by CD19(low)CD5(+) regulatory B cell subpopulation: characterisation of CD19 (high) and CD19(low) subpopulations of CD5(+) B cells. Yonsei Med J. 2011; 52:851–855.

13. Noh G, Lee JH. Regulatory B cells and allergic diseases. Allergy Asthma Immunol Res. 2011; 3:168–177.

14. Yanaba K, Bouaziz JD, Haas KM, Poe JC, Fujimoto M, Tedder TF. A regulatory B cell subset with a unique CD1dhiCD5+ phenotype controls T cell-dependent inflammatory responses. Immunity. 2008; 28:639–650.

15. Yang M, Deng J, Liu Y, Ko KH, Wang X, Jiao Z, et al. IL-10-producing regulatory B10 cells ameliorate collagen-induced arthritis via suppressing Th17 cell generation. Am J Pathol. 2012; 180:2375–2385.

16. Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988; 31:315–324.

17. Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, Bingham CO 3rd, et al. 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 2010; 62:2569–2581.

18. Prevoo ML, van't Hof MA, Kuper HH, van Leeuwen MA, van de Putte LB, van Riel PL. Modified disease activity scores that include twenty-eight-joint counts. Development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum. 1995; 38:44–48.

19. van Riel PL, van Gestel AM. Clinical outcome measures in rheumatoid arthritis. Ann Rheum Dis. 2000; 59:Suppl 1. i28–i31.

20. Calvo-Alén J, Corrales A, Sánchez-Andrada S, Fernández-Echevarría MA, Peña JL, Rodríguez-Valverde V. Outcome of late-onset rheumatoid arthritis. Clin Rheumatol. 2005; 24:485–489.

21. Tamas MM, Felea I, Rednic S. How much difference does the age at onset make in early arthritis patients? Comparison between the ACR 1987 and the ACR/EULAR 2010 classification criteria for rheumatoid arthritis at the time of diagnosis. Rheumatol Int. 2013; 33:2881–2884.

22. Ma L, Liu B, Jiang Z, Jiang Y. Reduced numbers of regulatory B cells are negatively correlated with disease activity in patients with new-onset rheumatoid arthritis. Clin Rheumatol. 2014; 33:187–195.

23. Jeong YI, Hong SH, Cho SH, Lee WJ, Lee SE. Induction of IL-10-producing CD1dhighCD5+ regulatory B cells following Babesia microti-infection. PLoS One. 2012; 7:e46553.
24. Ding Q, Yeung M, Camirand G, Zeng Q, Akiba H, Yagita H, et al. Regulatory B cells are identified by expression of TIM-1 and can be induced through TIM-1 ligation to promote tolerance in mice. J Clin Invest. 2011; 121:3645–3656.

25. MacDonald KP, Nishioka Y, Lipsky PE, Thomas R. Functional CD40 ligand is expressed by T cells in rheumatoid arthritis. J Clin Invest. 1997; 100:2404–2414.

26. Liu MF, Chao SC, Wang CR, Lei HY. Expression of CD40 and CD40 ligand among cell populations within rheumatoid synovial compartment. Autoimmunity. 2001; 34:107–113.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download