Abstract
Purpose
The purpose of this study was to compare once-daily tacrolimus with twice-daily tacrolimus in terms of safety, efficacy, and patient satisfaction.
Materials and Methods
This prospective, randomized, open-label, multicenter study was conducted at three institutes. Patients in the investigational group were converted from tacrolimus twice daily to the same dose of extended-release tacrolimus once daily at 1 month post-transplantation, while patients in the control group were maintained on tacrolimus twice daily. The efficacies, safeties, and patient satisfaction for the two drugs at 6 months post-transplantation were compared.
Results
Sixty patients were enrolled and randomized to the investigational group (28 of 29 patients completed the study) or the control group (26 of 31 patients completed the study). At 6 months post-transplantation, composite efficacy failure rates including the incidences of biopsy-confirmed acute rejection in the investigational and control groups were 0% and 10.7%, respectively; patient survival was 100% in each group. No difference in estimated glomerular filtration rate values were observed at 6 months post-transplantation (p=0.97). The safety and satisfaction profile (immunosuppressant therapy barrier scale) of once-daily tacrolimus was comparable with that of twice-daily tacrolimus (p=0.35).
Once-daily tacrolimus is an extended-release formulation of tacrolimus that can be administered once daily in the morning. Previous studies have demonstrated the non-inferiority of once-daily tacrolimus (Advagraf®, Astellas Pharma Inc., Tokyo, Japan) to twice-daily tacrolimus (Prograf®, Astellas Pharma Inc., Tokyo, Japan) in terms of its efficacy and safety for de novo renal transplant recipients. Studies have even demonstrated that kidney transplant recipients converted to once-daily tacrolimus could be safely maintained using the same therapeutic monitoring and patient care techniques used for twice-daily tacrolimus.1,2,3,4,5
Once-daily tacrolimus may improve adherence, resulting in better post-transplant outcomes. Successful outcomes after renal transplantation depend on patient adherence with immunosuppressive medications, because the effectiveness of medications in terms of preventing acute rejection after transplantation depend not only on the right choice of drugs, but also on active patient cooperation.6,7,8,9
However, prospective clinical trials have yet to assess conversion of twice-daily tacrolimus to once-daily tacrolimus for the specific same period.
The primary endpoint of this study was to determine the safety and efficacy of once-daily tacrolimus in kidney transplant recipients converted from twice-daily tacrolimus one month after transplantation. The secondary endpoint was to compare patient satisfaction between once-daily and twice-daily tacrolimus using the immunosuppressant therapy barrier scale (ITBS).
This open-label, randomized (1:1), double arm, multicenter, non-inferiority, conversion study was conducted at three transplant centers in Korea from April 2010 to June 2012. The study protocol was approved by the Institutional Review Board at all participating centers to ensure that study procedures were in accord with the Helsinki Declaration.
The study inclusion criteria were an age between 20 and 65 years and recipient of a primary kidney transplant from a deceased or living donor between 15 and 65 years of age. The study exclusion criteria were receipt or scheduled receipt of an organ transplant other than a kidney or scheduled receipt of a kidney from a non-heart beating or ABO blood group incompatible or lymphocyte cross match positive donor.
All recipients were administered twice-daily tacrolimus (Prograf®, Astellas Pharma Inc.,Tokyo, Japan) with prednisolone and mycophenolate mofetil until 1 month post-transplantation. Methyl-prednisolone was injected intravenously with the following doses: 500 mg on the day of operation and 250 mg on the day after, and corticosteroids were tapered to a maintenance dose of more than 5 mg a day (prednisolone or equivalent) orally twice or once a day. Target dose of mycophenolate mofetil was 1000-2000 mg/day, orally twice a day, for both groups throughout the study period. Tacrolimus (Prograf®) was initiated at 0.05-0.1 mg/kg, starting on the day before transplantation. Within a month after transplantation, dosages were individually adjusted to achieve a trough blood level between 5 and 15 ng/mL. Patients in the investigational group were converted to the same milligram-for-milligram daily dose of once-daily tacrolimus (Advagraf®, Astellas Pharma Inc., Tokyo, Japan) on the 28th day post-transplantation and were then maintained at tacrolimus trough levels of 3 to 12 ng/mL throughout the study. The patients in the control group continued to take twice-daily tacrolimus (Prograf®) at targeted blood trough levels of 3 to 12 ng/mL from 1 month post-transplantation.
Efficacy and safety analyses were performed using a modified intent-to-treat population composed of all randomized patients that received at least one dose of study drug. A composite primary efficacy endpoint was assessed: that is, efficacy failure rate at 6 months post-transplantation, including any mortality, graft failure (return to dialysis for >30 days or re-transplant), treatment for biopsy-confirmed acute rejection (BCAR), or loss to follow-up. The latter was defined as a lack of follow-up information for at least 3 months (90 days). Secondary endpoints incuded patient and graft survival rates at 6 months, incidences of BCAR at 1 and 6 months, estimated glomerular filtration rate (eGFR) as determined using the Nankivell method at 6 months,10 the amount of proteinuria in 24-hour urine at 6 months, creatinine clearance in 24-hour urine at 6 months, and the immunosuppressant therapy barrier scale (ITBS). Adverse events (AE) were recorded and coded during the study period. An adverse event was defined as any untoward medical occurrence, including an exacerbation of a per-existing condition, in a patient in a clinical investigation who received a pharmaceutical product. The event did not necessarily have a causal relationship with this treatment. The severity grade of AE was defined as: 1) mild, usually transient in nature and generally not interfering with normal activities; 2) moderate, sufficiently discomforting to interfere with normal activities; and 3) severe, prevents normal activities. Serious adverse events (SAEs) were defined as any AE undesirable signs, symptoms, or medical conditions that met any one of the following criteria: 1) was fatal or life-threatening, 2) resulted in persistent or significant disability/incapacity, 3) required hospitalization or prolongation of existing hospitalization, 4) was a congenital anomaly/birth defect, or 5) was an important medical event that might jeopardize the patient and might require medical or surgical intervention to prevent one of the outcomes listed above. Differences between adverse event incidences and the incidences of predefined potentially clinically significant laboratory values in the two study groups were assessed and analyzed.
ITBS is a 13-item scale that measures barriers to immunosuppressant adherence in transplant patients. ITBS was measured at 6 months post-transplantation. Each item was measured on a 5-point scale (1=strongly disagree, to 5=strongly agree) assessing respondent agreement with each statement. Items consisted of questions about how often: 1) they forgot to take their immunosuppressants, 2) were careless about taking their immunosuppressants, 3) stopped taking their immunosuppressants because they felt worse, and 4) missed taking their immunosuppressants for any reason.11
Study visits took place prior to transplantation (baseline) and at 1, 2, 4, and 6 months post-transplantation. At each visit, a complete physical examination was performed and laboratory values concerning kidney, liver, hematology, proteinuria, and tacrolimus in blood were measured. Blood pressure, weight, and any problems between visits were documented. All adverse and severe adverse events were documented.
All assessments of non-inferiority were made using a pre-specified margin of 25% with a 0.05 level of significance. Frequency distributions were plotted for categorical variables. The χ2 test or Fisher's exact test were used to compare the two treatment groups. Descriptive statistics were analyzed as continuous variables using Student's t-test. Continuous variables were expressed as means±standard deviations.
Based on reference to a previous study, the efficacy failure rates at post-transplant 6 months for the control and investigational groups were 14.0% and 15.1%, respectively.12 A sample size of 25 patients per each treatment group was calculated to have 80% power, 25% non-inferiority margin, and a 10% drop out rate. To allow for discontinuations, 60 patients (n=30 per group) were initially randomly assigned.
In total, 60 patients were enrolled in this study and randomized to either the investigational group (n=29) or the control group (n=31). Fifty-four (90.0%) patients remained on study medication at 6 months post-transplantation. One patient (3.4%) in the investigational group and 5 patients (16.1%) in the control group prematurely discontinued medication during follow-up. The reasons for premature discontinuations were adverse events (n=1), withdrawal of consent (n=3), graft loss (n=1), and protocol deviation (n=1). Donor and recipient characteristics were comparable in the two study groups (Table 1).
Composite efficacy failure rates including the incidence of biopsy-confirmed acute rejection until 6 months post-transplantation were 0% in the investigational group and 10.7% in the control group, and the investigational group was statistically non-inferior to the control group. One graft loss in the control group and no patient death in either group were recorded. No statistical differences were observed between the groups in terms of Kaplan-Meier estimates of patient proportions free of composite efficacy failure at 6 months post-transplantation (100% in the investigational group and 89.3% in the control group; p=0.236).
The incidences of treated BCAR within 6 months of transplantation were not significantly different between the two groups: 0% in the investigational group and 10.7% in the control group (p=0.075), which was statistically non-inferior to the investigational group.
Mean eGFRs (by the Nankivell method) in the investigational group were 40.2±6.9 mL/min/1.73 m2 at 1 month, 41.7±6.3 mL/min/1.73 m2 at 2 months, 41.2±6.0 mL/min/1.73 m2 at 4 months, and 41.8±6.1 mL/min/1.73 m2 at 6 months post-transplantation, and in the control group were 41.0±5.2 mL/min/1.73 m2 at 1 month, 41.6±5.3 mL/min/1.73 m2 at 2 months, 42.5±5.9 mL/min/1.73 m2 at 4 months, and 41.9±5.8 mL/min/1.73 m2 at 6 months post-transplantation. Group renal functions were not statistically different at any time (p>0.05).
Measured creatinine clearance by 24-hour urine collection at 6 months post-transplantation were similar between the two groups (64.1±21.5 mL/min/1.73 m2 in the investigational group and 63.64±21.3 mL/min/1.73 m2 in the control group; p=0.933).
Excreted protein amounts at 6 months post-transplantation were similar at 104.3±84.4 and 91.4±83.4 mg per day in the investigational and control groups (p=0.589).
At follow-up visits, most subjects did not require dose adjustment. The mean daily doses and whole blood concentrations of tacrolimus are summarized in Table 2.
Overall incidences of adverse events at 1 month post-transplantation were comparable between the two groups (71.4% of patients in the investigational group and 75.0% in the control group; p=0.763), and incidences of SAEs were also comparable (14.7% in the investigational group and 15.5% in the control group; p=0.415). The incidences of adverse events by system organ class were generally similar between the groups, and most were mild-to-moderate in severity (Table 3).
As far as we know, there has not been a prospective trial designed to assess conversion from twice-daily tacrolimus to once-daily tacrolimus at the same point in time, one month after transplantation, which is the main reason why we conducted this trial. Because many transplant doctors are accustomed to using twice-daily tacrolimus and maintaining a dose of tacrolimus that is usually determined at one month post-transplantation, we considered one month after transplantation as an optimal timing for conversion of twice-daily tacrolimus to once-daily tacrolimus. The results of the present clinical trial show that the once-daily tacrolimus is effective with good tolerability and safety when administered from 1 month after transplantation. In the present study, during the 6-month post-transplantation period, no graft was lost and no patient death occurred in the investigational group. Furthermore, no episode of biopsy-proven acute rejection was encountered in the investigational group.
Despite the finding that once-daily tacrolimus provided more stable drug blood concentrations than twice-daily tacrolimus,13 it was found to be necessary to use higher doses of once-daily tacrolimus to reach targeted trough levels after conversion from twice-daily tacrolimus, which concurs with previous reports.14,15,16,17 As shown in the results, administered doses of once-daily tacrolimus were higher than those of twice-daily tacrolimus after conversion, and the mean blood trough levels of once-daily tacrolimus were lower than those of twice-daily tacrolimus, especially at post-transplant 4 and 6 months. The rate of increasing tacrolimus dose was higher in the once-daily tacrolimus group than twice-daily tacrolimus group. Therefore, in order to prevent development of acute rejection, especially during the early post-transplant period, blood trough levels should be cautiously monitored when twice-daily tacrolimus is converted to once-daily. A recent report revealed another pharmacokinetic difference between once-daily and twice-daily tacrolimus regarding the intravariability of blood trough concentration (C0), which was significantly lower for once-daily tacrolimus.18
Although the present study was not powered to test for superiority, the BCAR rate at 6 months was lower for once-daily tacrolimus. This finding suggests that the efficacy of once-daily tacrolimus is at least equivalent to that of twice-daily tacrolimus. Only when the blood trough levels are appropriately maintained, tacrolimus-based immunosuppression should be enough to suppress acute rejection regardless of its frequency of dosing (once-daily or twice-daily). Previous studies have failed to demonstrate the equivalence of the efficacy of once-daily versus twice-daily tacrolimus in de novo kidney transplant patients.3,4 In the present study, we initiated immunosuppression with twice-daily tacrolimus during this critical period, and then converted to once-daily tacrolimus. This conversion at 1 month post-transplantation may have avoided the risk of acute rejection immediately after transplantation, because this period is vulnerable to development of acute rejection. In addition, it is easier to adjust optimal blood trough levels using twice-daily tacrolimus than once-daily tacrolimus.
Generally, adherence has been reported to increase from 59% for 3 times daily dosing regimens to 83% for once daily regimens,19 which leads to the conclusion that reducing the dosage frequency is one of the most important means of improving adherence. However, all kidney transplant patients enrolled in this study were taking adjunctive immunosuppressants, such as glucocorticoid and mycophenolate mofetil, which required twice-daily dosing. We believe that this explains why ITBS scores were not found to differ significantly in our two groups (p=0.345). The limitations of this study include that it could not provide evidence of drug adherence associated with the dosage frequency and that it failed to demonstrate only once-daily drug as statistically superior for this study population who were taking concomitant medications twice a day.
In conclusion, conversion to once-daily tacrolimus at 1 month after kidney transplantation was found to be non-inferior in terms of efficacy and safety to twice-daily tacrolimus. In terms of patient satisfaction, despite once-daily tacrolimus would be partly useful to reducing the frequency of dosing, it failed to be statistically superior for this study population who were taking concomitant medications twice a day.
Figures and Tables
Table 1
Baseline Demographics and Clinical Characteristics of the Study Population (ITT Population)
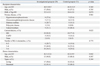
ITT, intention-to-treat; BMI, body mass index; HD, hemodialysis; CAPD, continuous ambulatory peritoneal dialysis; HLA, human leukocyte antigen.
Continuous variables are expressed as the mean±standard deviation (SD) and their p-values were calculated with t-test. Categorical variables are expressed as number (%) and their p-values were calculated with Fisher's exact test.
ACKNOWLEDGEMENTS
This corporation-initiated trial (CIT) was funded by Astellas Pharma Korea, Inc. This study was registered with www.clinicaltrials.gov; registration identifier=NCT01742624.
Yu Seun Kim has received grants from Novartis, Astellas, Wyeth, Roche.
References
1. Alloway R, Steinberg S, Khalil K, Gourishankar S, Miller J, Norman D, et al. Two years postconversion from a prograf-based regimen to a once-daily tacrolimus extended-release formulation in stable kidney transplant recipients. Transplantation. 2007; 83:1648–1651.

2. Alloway R, Steinberg S, Khalil K, Gourishankar S, Miller J, Norman D, et al. Conversion of stable kidney transplant recipients from a twice daily Prograf-based regimen to a once daily modified release tacrolimus-based regimen. Transplant Proc. 2005; 37:867–870.

3. Krämer BK, Charpentier B, Bäckman L, Silva HT Jr, Mondragon-Ramirez G, Cassuto-Viguier E, et al. Tacrolimus once daily (ADVAGRAF) versus twice daily (PROGRAF) in de novo renal transplantation: a randomized phase III study. Am J Transplant. 2010; 10:2632–2643.

4. Silva HT Jr, Yang HC, Abouljoud M, Kuo PC, Wisemandle K, Bhattacharya P, et al. One-year results with extended-release tacrolimus/MMF, tacrolimus/MMF and cyclosporine/MMF in de novo kidney transplant recipients. Am J Transplant. 2007; 7:595–608.

5. Guirado L, Cantarell C, Franco A, Huertas EG, Fructuoso AS, Fernández A, et al. Efficacy and safety of conversion from twice-daily to once-daily tacrolimus in a large cohort of stable kidney transplant recipients. Am J Transplant. 2011; 11:1965–1971.

6. Butler JA, Roderick P, Mullee M, Mason JC, Peveler RC. Frequency and impact of nonadherence to immunosuppressants after renal transplantation: a systematic review. Transplantation. 2004; 77:769–776.

7. Weng FL, Israni AK, Joffe MM, Hoy T, Gaughan CA, Newman M, et al. Race and electronically measured adherence to immunosuppressive medications after deceased donor renal transplantation. J Am Soc Nephrol. 2005; 16:1839–1848.

9. Regazzi MB, Alessiani M, Rinaldi M. New strategies in immunosuppression. Transplant Proc. 2005; 37:2675–2678.

10. Nankivell BJ, Gruenewald SM, Allen RD, Chapman JR. Predicting glomerular filtration rate after kidney transplantation. Transplantation. 1995; 59:1683–1689.

11. Chisholm MA, Lance CE, Williamson GM, Mulloy LL. Development and validation of an immunosuppressant therapy adherence barrier instrument. Nephrol Dial Transplant. 2005; 20:181–188.

12. Cross SA, Perry CM. Tacrolimus once-daily formulation: in the prophylaxis of transplant rejection in renal or liver allograft recipients. Drugs. 2007; 67:1931–1943.

13. Kurnatowska I, Krawczyk J, Oleksik T, Nowicki M. Tacrolimus dose and blood concentration variability in kidney transplant recipients undergoing conversion from twice daily to once daily modified release tacrolimus. Transplant Proc. 2011; 43:2954–2956.

14. de Jonge H, Kuypers DR, Verbeke K, Vanrenterghem Y. Reduced C0 concentrations and increased dose requirements in renal allograft recipients converted to the novel once-daily tacrolimus formulation. Transplantation. 2010; 90:523–529.

15. Jelassi ML, Lefeuvre S, Karras A, Moulonguet L, Billaud EM. Therapeutic drug monitoring in de novo kidney transplant receiving the modified-release once-daily tacrolimus. Transplant Proc. 2011; 43:491–494.

16. Crespo M, Mir M, Marin M, Hurtado S, Estadella C, Gurí X, et al. De novo kidney transplant recipients need higher doses of Advagraf compared with Prograf to get therapeutic levels. Transplant Proc. 2009; 41:2115–2117.

17. Hougardy JM, Broeders N, Kianda M, Massart A, Madhoun P, Le Moine A, et al. Conversion from Prograf to Advagraf among kidney transplant recipients results in sustained decrease in tacrolimus exposure. Transplantation. 2011; 91:566–569.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download