Abstract
Purpose
To find out the possible role of 1,25(OH)2 vitamin D3 [1,25(OH)2D3] and parathyroid hormone (PTH) as intrinsic factors in urinary calcium stone formers (SFs), we investigated their relationship with serum and urinary biochemical parameters.
Materials and Methods
A total of 326 calcium SFs (male: 204, female: 122) were enrolled and underwent outpatient metabolic evaluations including 1,25(OH)2D3 and PTH as well as serum and 24-hour urinary biochemical parameters. As control, 163 age- and sex-matched (2:1) individuals (non-SFs) who have never urinary stone episode were included.
Results
1,25(OH)2D3 level was positively correlated with urinary calcium excretion (r=0.347, p<0.001). The hypercalciuric group and recurrent SFs had higher serum 1,25(OH)2D3 levels than the normocalciuric group (p<0.001) and first SFs (p=0.050). In the adjusted multiple linear regression analysis, serum 1,25(OH)2D3 level (β=0.259, p<0.001) and serum PTH level (β=-0.160, p<0.001) were significantly correlated with urinary calcium excretion. The patients in highest tertile of 1,25(OH)2D3 had a more than 3.1 fold risk of hypercalciuria than those in the lowest tertile (odds ratio=3.14, 95% confidence interval: 1.431-6.888, p=0.004). No correlation was observed between PTH and 1,25(OH)2D3 (R=0.005, p=0.929) in calcium SFs, while a negative correlation was found in controls (R=-0.269, p=0.001).
Conclusion
1,25(OH)2D3 was closely correlated with urinary calcium excretion, and high 1,25(OH)2D3 levels were detected in the hypercalciuric group and in recurrent SFs. However, 1,25(OH)2D3 was not correlated with PTH in calcium SFs. These findings suggest that 1,25(OH)2D3 might be important intrinsic factor for altered calcium regulation in SFs.
The incidence of urinary stone formation has been increasing recently, and the lifetime risk of stone formation is estimated at 5-12% in Europe and the USA.1,2,3 Environmental, genetic, nutritional, intrinsic, anatomic and metabolic factors contribute to urinary stone formation.4,5,6,7,8 Hypercalciuria, regardless of its underlying mechanism, is the most common metabolic abnormality in patients with calcium stones.9,10 Calciuria is a net loss of calcium in the urine after renal reabsorption and the final result of numerous regulatory processes.11 Parathyroid hormone (PTH) and 1,25-dihydroxy vitamin D3 [1,25(OH)2D3] are considered to be the endocrine regulators of calcium homeostasis. PTH secretion, which is triggered by hypocalcemia, increases extracellular calcium levels by stimulating bone resorption, renal reabsorption, and intestinal calcium absorption indirectly through the synthesis of 1,25(OH)2D3 in the kidney.12 Due to their role in the control of calcium levels, PTH and 1,25(OH)2D3 have received much attention. However, the exact roles of these intrinsic factors in urolithiasis remain to be elucidated. The aim of this study was to find the association between PTH or 1,25(OH)2D3 and serum and urinary metabolites and the correlation between PTH or 1,25(OH)2D3 and urinary calcium excretion in calcium stone formers (SFs).
Between 2009 and 2011, 326 calcium SFs (male: 204, female: 122) with informed consent agreement were enrolled. Pediatric patients (<16 years) and patients with incomplete 24 hour urine collection, impaired renal function (serum creatinine >1.5 mg/dL), infection stones, radiolucent stones, malformation of the urological system, hypercalcemia, prior bowel surgery, or a prior diagnosis of primary hyperparathyroidism or other systemic diseases (any cancer, alcoholic liver disease and osteoporosis drug medication like calcium pills etc.), that might affect calcium and bone metabolism were excluded. Controls were selected with similar age and gender proportions to the calcium SFs, and subjects were screened to ensure that they were within the normal range of all laboratory findings and had no history of urinary stone. The Ethics Committee of our institution approved this protocol. The collection and analysis of all samples was approved by the Institutional Review Board of our institution. The data collected included the history of kidney stones and medications, and a metabolic evaluation such as 24-hour urinary and fasting serum biochemistry as well as intact PTH and 1,25(OH)2D3 which was performed at the same time. Intact PTH was measured with an immunoradiometric assay with an ELSA-PTH kit (CIS Bio International, Paris, France), and 1,25(OH)2D3 levels were also measured with a radioimmunoassay with a 1,25(OH)2D3 RIA kit (Immunodiagnostic Systems Ltd., Boldon Colliery, Tyne & Wear, UK). The metabolic evaluation was performed at least 4-6 weeks after returning to their normal life. SFs were advised to continue their usual diet, and none were placed on a low calcium diet or preventive medications. Patients were divided into two groups according to urinary calcium excretion (hypercalciuria vs. normocalciuria) and the prior stone episode (first time vs. recurrent), respectively, and the clinical and laboratory characteristics of each group were compared. Hypercalciuria was defined as 24 h urinary calcium excretion of more than 300 mg per 24 hour in men and 250 mg per 24 hour in women. Hypercalciuric SFs and normocalciuric SFs were 19.9% (65/326) and 80.1% (261/326), while the fractions of first SFs and recurrent SFs were 57.1% and 42.9%, respectively (total 324 patents were analyzed due to 2 missing values). To compare 1,25(OH)2D3 and PTH levels between calcium SFs and controls, 163 age- and sex-matched controls were included.
Clinical characteristics, serum laboratory parameters including PTH and 1,25(OH)2D3, and urinary biochemical parameters were compared in each group. The correlation between PTH or 1,25(OH)2D3 and serum and urinary parameters was assessed by the Spearman correlation test. The relationship between PTH and 1,25(OH)2D3 in calcium SFs and controls was assessed by the linear regression test. The differences in serum and urinary metabolites between subgroups were assessed by the Student's t-test. Multiple linear regression analysis was performed to evaluate the association between 24 h urinary calcium excretion and serum 1,25(OH)2D3 or PTH after adjusting for the effects of other parameters. The logistic regression was carried out to identify the association between hypercalciuria and tertiles of serum 1,25(OH)2D3. Dependent variable was the hypercalciuria; independent variable was tertile of serum 1,25(OH)2D3. Tertile cut-off points were determined on variable distribution of patients in the full study group. Odds ratios (ORs) and 95% confidential intervals (CIs) were estimated using logistic regression models. Statistical analysis was performed using SPSS 19.0 software (SPSS Inc., Chicago, IL, USA). A p-value less than 0.05 was considered statistically significant.
The mean age of the study population was 45.8±12.3 years and the mean body mass index (BMI) was 24.5±3.4 kg/m2. The mean serum calcium, phosphate, uric acid, PTH, and 1,25(OH)2D3 were 9.49±0.49 mg/dL, 3.61±0.62 mg/dL, 5.65±1.52 mg/dL, 28.0±14.4 pg/mL, and 52.5±19.0 ng/mL, respectively.
1,25(OH)2D3 levels were positively correlated with 24 h urinary phosphate (r=0.120, p=0.030), uric acid (r=0.158, p=0.004), pH (r=0.170, p=0.002), and calcium excretion (r=0.347, p<0.001). However, in the multiple linear regression analysis, the 1,25(OH)2D3 level was correlated significantly only with 24 h urine calcium excretion (β=0.355, p<0.001).
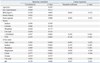
PTH was positively correlated with age (r=0.146, p=0.008) and sex (r=0.148, p=0.008), but inversely correlated with 24 h urinary magnesium (r=-0.116, p=0.036) and calcium excretion (r=-0.193, p<0.001). In the multiple linear regression analysis, PTH level was inversely correlated with 24 h urinary calcium excretion (β=-0.188, p<0.001) (Table 1).
There were no significant differences in parameters such as age, serum calcium, phosphate and uric acid between the hypercalciuria group and the normocalciuria group, or between first SFs and recurrent SFs. The serum 1,25(OH)2D3 level was significantly higher in the hypercalciuric group and recurrent SFs than in the normocalciuric group and first SFs (p<0.001, p=0.050, respectively). However, there were no significant differences in PTH levels between these groups (p=0.295, 0.256, respectively) (Table 2).
Urinary calcium excretion was positively correlated with BMI (r=0.149, p=0.007), stone episodes (r=0.151, p=0.006), 1,25(OH)2D3 level (r=0.347, p<0.001), 24 h urine total volume (r=0.305, p<0.001), urinary sodium (r=0.502, p<0.001), phosphate (r=0.469, p<0.001), magnesium (r=0.375, p<0.001), citrate (r=0.277, p<0.001), uric acid excretion (r=0.553, p<0.001), and pH (r=0.170, p=0.002), but was inversely correlated with serum PTH level (r=-0.193, p<0.001). To evaluate the association between 24 h urinary calcium excretion and serum vitamin D3 or PTH after adjusting for the effects of other parameters, multiple linear regression analysis was performed using parameters correlated with urinary calcium excretion in Pearson correlation. Stone episodes (β=0.083, p=0.045), serum 1,25(OH)2D3 (β=0.259, p<0.001) and PTH (β=-0.160, p<0.001) levels, and urinary sodium (β=0.270, p<0.001), phosphate (β=0.192, p<0.001) and uric acid (β=0.243, p<0.001) levels, were significantly associated with calcium excretion (Table 3). The patients in highest tertile of 1,25(OH)2D3 had a more than 3.1 fold risk of hypercalciuria than those in the lowest tertile (OR=3.14, 95% CI: 1.431-6.888, p=0.004) (Table 4).
The mean PTH and 1,25(OH)2D3 were 28.0±14.4 pg/mL, 52.5±19.0 ng/mL in calcium SFs and 27.8±10.8 pg/mL, 18.4±7.3 ng/mL in controls (p=0.871, p<0.001, respectively). 1,25(OH)2D3 levels were significantly higher in calcium SFs than in controls (p<0.001). No correlation was found between 1,25(OH)2D3 and PTH in calcium SFs (R=0.005, p=0.929). In controls, the 1,25(OH)2D3 levels were inversely correlated with PTH levels (R=-0.269, p=0.001) (Fig. 1).
In the present study, an assessment of the roles of 1,25(OH)2D3 and PTH in calcium stone formers revealed a correlation between 1,25(OH)2D3 and PTH levels and urinary calcium excretion. 1,25(OH)2D3 levels were significantly increased in calcium SFs compared to controls, and the balance between 1,25(OH)2D3 and PTH was altered in calcium SFs.
Hypercalciuria is the most common metabolic abnormality in patients with urolithiasis.10 Calcium oxalate overgrowth on plaque is due to calcium oxalate supersaturation, which is strongly linked to hypercalciuria.13 The present data showing a strong correlation between serum 1,25(OH)2D3 and PTH levels and urinary calcium excretion suggested that urinary calcium excretion might be affected by intrinsic factors such as serum 1,25(OH)2D3 and PTH, as well as environment factors such as sodium intake.
Calcium regulates a wide range of biological processes and is one of the principal constituents of bone. The maintenance of adequate concentrations of calcium in the extracellular fluid requires the activity of two hormones, 1,25(OH)2D3 and PTH.12 PTH, which functions through a negative feedback loop to regulate extracellular calcium levels, is secreted in response to hypocalcemia and stimulates the release of calcium from bone, decreases the urinary loss of calcium, and indirectly stimulates calcium absorption in the small intestine by stimulating the synthesis of 1,25(OH)2D3.12
The relationship between 25(OH) vitamin D3 (calcidiol) levels and hypercalciuria has been reported previously.14,15 However, some studies reported a weak correlation between calcium excretion and the level of 25(OH) vitamin D3.16 In reality, 1,25(OH)2D3 is considered to be more important compared to 25(OH)D3 for mediating the biological actions of vitamin D on calcium and bone metabolism.17 In the present study, a strong relationship between 1,25(OH)2D3 and urinary calcium excretion was observed. Moreover, the hypercalciuric group had higher levels of 1,25(OH)2D3 than the normocalciuric group. Hess and Jaeger18 reported that patients with idiopathic hypercalciuria had higher serum concentrations of 1,25(OH)2D3 than normocalciuric SFs. Furthermore, in the present study, 1,25(OH)2D3 was significantly increased in calcium SFs compared to controls. Shakhssalim, et al.19 also reported that the serum 1,25(OH)2D3 levels in renal SFs were higher than in the control groups.
PTH stimulates the metabolism of 1,25(OH)2D3 to its active hormonal form, 1,25(OH)2D3 in the kidney. 1,25(OH)2D3 promotes the absorption of calcium in the small intestine and calcium resorption in bone.20 Although the functions of 1,25(OH)2D3 and PTH are closely associated, a correlation between the levels of 1,25(OH)2D3 and PTH was not observed in the current study. Alterations in the balance between PTH and 1,25(OH)2D3 could be an important factor in calcium stone formation in calcium SFs.
PTH also correlated negatively with urinary calcium excretion. However, based on the exclusion of patients with hyperparathyroidism, this correlation was considered to be part of the normal process of homeostasis of hypercalciuria. Hess and Jaeger18 reported that primary intestinal hyperabsorption of calcium led to the depression of PTH secretion and PTH level was lower in hypercalciuric SFs compared to normocalciuric SFs at the same blood Ca2+ levels.21 Similarly, PTH was lower in hypercalciuric SFs than in normocalciuric SFs in the current study, although the difference between the two groups did not reach statistical significance.
In the present study, multiple regression analysis revealed a positive correlation between 1,25(OH)2D3 and urinary calcium excretion. Shakhssalim, et al.19 also reported that 1,25(OH)2D3 was an important hormone in the pathogenesis of recurrent renal calcium stone disease and could increase the risk of renal stone development by increasing urinary calcium and phosphorus excretion. However, no studies addressing the correlation between 1,25(OH)2D3 and PTH in calcium SFs have been published to date. In our study, 1,25(OH)2D3 level was positively correlated with urinary calcium excretion and higher in calcium SFs than in controls. However, the association between 1,25(OH)2D3 and PTH in calcium SFs was not found. Presumably, these findings suggest that altered regulation of 1,25(OH)2D3 rather than PTH might be the primary intrinsic factor for pathogenesis in calcium SFs.
There are several limitations to the present study. First, absorptive, renal induced, and resorptive hypercalciuria are common types of hypercalciuria.22 The exclusion of patients with primary hyperparathyroidism from the study resulted in the exclusion of resorptive hypercalciuria. However, renal induced hypercalciuria could not be excluded because we did not perform a calcium load test. Nevertheless, due to the low prevalence of renal hypercalciuria, the difference is considered to be insignificant. Second, occupation and seasonal variation were not considered in this study despite the importance of the effect of skin exposure to sunlight on 1,25(OH)2D3 levels. Parry and Lister23 reported increased exposure to sunlight as the most likely cause of hypercalciuria. Third, This study was performed by cross-sectional manner and therefore, causality cannot be determined. It may be the idiopathic hypercalciuria which is causing the increasing the 1,25(OH)2D3, and not the other way around. However, this would be unlikely seen from our result that PTH was actually negatively associated with urinary calcium excretion. Finally, these results alone cannot reach the mechanism by what calcium SFs have elevated 1,25(OH)2D3 and future studies will need to be considered.
In conclusion, the present study is the first to examine the relationship between serum 1,25(OH)2D3 and PTH in calcium SFs. 1,25(OH)2D3 was closely correlated with urinary calcium excretion levels. Increased 1,25(OH)2D3 levels were observed in the hypercalciuric group as well as in recurrent SFs. In calcium SFs, the 1,25(OH)2D3 level was high and was not correlated with PTH levels. These findings suggest that 1,25(OH)2D3 might be an important intrinsic factor in calcium stone formation.
Figures and Tables
Fig. 1
Correlation between 1,25(OH)2D3 and PTH (A) in calcium stone formers and (B) in controls. 1,25(OH)2D3, 1,25-dihydroxy vitamin D3; PTH, parathyroid hormone.

Table 1
Relationships between Serum 1,25(OH)2D3 or PTH Levels and Clinical and Laboratory Parameters
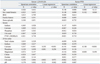
Table 2
Comparison of Clinical Characteristics and Serum Parameters According to Calciuria and Stone Episodes

ACKNOWLEDGEMENTS
This research was supported by a National Research Foundation of Korea (NRF) grant funded by the Korea government (MEST) (2012-0000476) and by a grant from the Next-Generation BioGreen 21 Program (PJ.009621), Rural Development Administration, Republic of Korea. The biospecimens for this study were provided by the Chungbuk National University Hospital, a member of the National Biobank of Korea, which is supported by the Ministry of Health, Welfare and Family Affairs. All samples derived from the National Biobank of Korea were obtained with informed consent under Institutional Review Board-approved protocols.
References
1. Bartoletti R, Cai T, Mondaini N, Melone F, Travaglini F, Carini M, et al. Epidemiology and risk factors in urolithiasis. Urol Int. 2007; 79:Suppl 1. 3–7.

2. Stamatelou KK, Francis ME, Jones CA, Nyberg LM, Curhan GC. Time trends in reported prevalence of kidney stones in the United States: 1976-1994. Kidney Int. 2003; 63:1817–1823.

4. Griffin DG. A review of the heritability of idiopathic nephrolithiasis. J Clin Pathol. 2004; 57:793–796.

5. Mossetti G, Rendina D, Viceconti R, Manno G, Guadagno V, Strazzullo P, et al. The relationship of 3' vitamin D receptor haplotypes to urinary supersaturation of calcium oxalate salts and to age at onset and familial prevalence of nephrolithiasis. Nephrol Dial Transplant. 2004; 19:2259–2265.

6. Relan V, Khullar M, Singh SK, Sharma SK. Association of vitamin D receptor genotypes with calcium excretion in nephrolithiatic subjects in northern India. Urol Res. 2004; 32:236–240.

7. Scott P, Ouimet D, Valiquette L, Guay G, Proulx Y, Trouvé ML, et al. Suggestive evidence for a susceptibility gene near the vitamin D receptor locus in idiopathic calcium stone formation. J Am Soc Nephrol. 1999; 10:1007–1013.

8. Smith LH. Pathogenesis of renal stones. Miner Electrolyte Metab. 1987; 13:214–219.
9. Bushinsky DA, Parker WR, Asplin JR. Calcium phosphate supersaturation regulates stone formation in genetic hypercalciuric stone-forming rats. Kidney Int. 2000; 57:550–560.

10. Coe FL, Parks JH, Asplin JR. The pathogenesis and treatment of kidney stones. N Engl J Med. 1992; 327:1141–1152.

11. Pasch A, Frey FJ, Eisenberger U, Mohaupt MG, Bonny O. PTH and 1.25 vitamin D response to a low-calcium diet is associated with bone mineral density in renal stone formers. Nephrol Dial Transplant. 2008; 23:2563–2570.

12. Goodman HM. Hormonal regulation of calcium balance. In : Goodman HM, editor. Basic medical endocrinology. 4th ed. London: Elsevier;2009. p. 197–218.
13. Kuo RL, Lingeman JE, Evan AP, Paterson RF, Parks JH, Bledsoe SB, et al. Urine calcium and volume predict coverage of renal papilla by Randall's plaque. Kidney Int. 2003; 64:2150–2154.

14. Berlin T, Björkhem I, Collste L, Holmberg I, Wijkström H. Relation between hypercalciuria and vitamin D3-status in patients with urolithiasis. Scand J Urol Nephrol. 1982; 16:269–273.
15. Elomaa I, Karonen SL, Kairento AL, Pelkonen R. Seasonal variation of urinary calcium and oxalate excretion, serum 25(OH)D3 and albumin level in relation to renal stone formation. Scand J Urol Nephrol. 1982; 16:155–161.
16. Berlin T, Emtestam L, Björkhem I. Studies on the relationship between vitamin D3 status and urinary excretion of calcium in healthy subjects: effects of increased levels of 25-hydroxyvitamin D3. Scand J Clin Lab Invest. 1986; 46:723–729.

17. Zerwekh JE. Vitamin D metabolism and stones. In : Rao PN, Preminger GM, Kavanagh JP, editors. Urinary Tract Stone Disease. London: Springer;2011. p. 169–179.
18. Hess B, Jaeger P. The tale of parathyroid function in idiopathic hypercalciuria. Scanning Microsc. 1993; 7:403–408.
19. Shakhssalim N, Gilani KR, Parvin M, Torbati PM, Kashi AH, Azadvari M, et al. An assessment of parathyroid hormone, calcitonin, 1,25 (OH)2 vitamin D3, estradiol and testosterone in men with active calcium stone disease and evaluation of its biochemical risk factors. Urol Res. 2011; 39:1–7.

20. Yu ASL. Renal transport of calcium, magnesium, and phosphate. In : Brenner BM, editor. Brenner and Rector's Kidney. Philadelphia: WB Saunders;2004. p. 535–572.
21. Hess B, Casez JP, Takkinen R, Ackermann D, Jaeger P. Relative hypoparathyroidism and calcitriol up-regulation in hypercalciuric calcium renal stone formers--impact of nutrition. Am J Nephrol. 1993; 13:18–26.

22. Pearle MS, Pak CY. Renal calculi: a practical approach to medical evaluation and management. In : Andreucii VE, Fine LG, editors. International Yearbook of Nephrology. New York: Oxford University Press;1996. p. 69–80.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download