Abstract
Purpose
To achieve maximal safe resection during brain tumor surgery, functional image-merged neuronavigation is widely used. We retrospectively reviewed our cases in which diffusion tensor image (DTI)-merged functional neuronavigation was performed during surgery.
Materials and Methods
Between November 2008 and May 2010, 123 patients underwent surgery utilizing DTI-merged neuronavigation. Anatomical magnetic resonance images (MRI) were obtained preoperatively and fused with DTI of major white matter tracts, such as the corticospinal tract, optic radiation, or arcuate fasciculus. We used this fused image for functional neuronavigation during brain tumor surgery of eloquent areas. We checked the DTI images together with postoperative MRI images and evaluated the integrity of white matter tracts.
Results
A single white matter tract was inspected in 78 patients, and two or more white matter tracts were checked in 45 patients. Among the 123 patients, a grossly total resection was achieved in 90 patients (73.2%), subtotal resection in 29 patients (23.6%), and partial resection in 4 patients (3.3%). Postoperative neurologic outcomes, compared with preoperative function, included the following: 100 patients (81.3%) displayed improvement of neurologic symptoms or no change, 7 patients (5.7%) experienced postoperative permanent neurologic deterioration (additional or aggravated neurologic symptoms), and 16 patients (13.0%) demonstrated transient worsening.
Although the relationships between extent of resection and clinical outcomes have been debated for a long time, there is no disagreement on maximal safe resection as a goal of brain tumor surgery. Many noninvasive modalities, such as functional magnetic resonance images (MRI) and diffusion tensor image (DTI), to identify eloquent areas have been widely used over the past few decades. More recently, many attempts have been made to fuse pre-surgical functional images with anatomical images, known as functional neuronavigation.1,2,3,4 DTI is a relatively new method that can demonstrate the orientation and integrity of major white matter fibers in vivo,2,5,6 and its combination with anatomical neuronavigation has been used in image-guided surgery of lesions adjacent to eloquent areas to avoid neurologic deteriorations after surgery.7,8,9 Integration of two studies (anatomical and functional images) can be difficult or time-consuming; however, commercial computing programs have made this an easy and quick process. We started to use commercial software for fiber tracking and image fusion in our institute. The aim of this study was to investigate the efficacy and limitations of DTI-merged functional neuronavigation in brain surgery.
We retrospectively reviewed 123 patients (63 males and 60 females; mean age 48.6 years, range 18-78 years) who underwent surgery using DTI-merged functional navigation. We used functional neuronavigation in cases of surgery for lesions adjacent to essential white matter tracts, such as the corticospinal tract, optic radiation, or arcuate fasciculus. We performed an awake craniotomy for brain mapping in 22 cases (17.9%).
Anatomical datasets were acquired on a 3T whole-body MR scanner (Magnetom Trio, Siemens Medical Solutions, Erlangen, Germany) using a T2-weighted turbo spin-echo (TSE) or contrast-enhanced three-dimensional (3D) T1-weighted magnetization-prepared rapid-gradient-echo (MP-RAGE) sequence. The parameters were as follows: TR=3600 ms; TE=86 ms; flip angle=160°; field of view (FOV)=230×184.8 mm; 288×448 matrix; 70 coronal slices and a slice thickness of 2 mm for T2-weighted TSE sequences or TR=2300 ms; TE=2.98 ms; inversion time=900 ms; flip angle=9°; FOV=256×256 mm; 184×256 matrix with 1×1×1 mm spatial resolution; 224 coronal slices and a slice thickness of 1 mm for 3D T1-weighted MP-RAGE sequences.
DTI was performed using a single-shot spin echo-echo planar imaging sequence. DTI parameters were as follows: TE=76 ms; TR=6599-8280 ms; zero-filled to 128 matrices over a 224×224 mm FOV; section thickness of 2 mm without a gap, and b=600 s/mm2 with 32 directions.
All image data were transferred via intranet to the planning computer. After loading of the DTI source images, several processing steps were needed to build a DTI color map, which included gradient assignment, gradient registration, and tensor computation. All the sequences were generated by commercial software (Stealth VIZ™ with Stealth DTI system, Medtronic, MN, USA) automatically. The DTI was presented as color-encoded fractional anisotropy (FA) maps. Tract seeding was performed by defining a rectangular volume of interest (VOI) in FA maps, which co-registered standard anatomic datasets. The definition of the VOI depended on the fiber structures to be displayed. A single, experienced neurosurgeon (JM, Cho) with knowledge of the fiber pathways placed the single or multiple VOIs on the color maps. For each lesion, we drew one or more VOI for the corticospinal tract, optic radiation, or arcuate fasciculus. If a more specific fiber bundle was needed, we designated a mid-point VOI located in the middle of the course of an interesting tract. The fibers can be retained, excluded, or deleted. For example, in case of the corticospinal tract, a seed point was usually placed on the posterior limb of the internal capsule and a midpoint was placed on the lateral part of the medulla. Tracking was initiated for the seed point in both forward and backward directions. The default value of angulation threshold was 45 degrees and the FA threshold was 0.2.
After tracking, the fibers were stored as an analyzed image and header file for import into the navigation system. Three separate image sets were stored as separate files (fiber dataset only, navigation dataset only, and fused data set). Then, these images were transferred to the operating room via intercomputer network or a compact disc.
A traditional navigation system (Stealth Treon, Medtronic, MN, USA) was used for the operation and image fusion. Before surgery, pre-built DTI-merged neuronavigation images were registered from previous datasets. For each patient's registration, five or more adhesive fiducial skin markers were placed in a scattered pattern on the head before image acquisition, and we registered those markers with a pointer after their position was defined in the 3D dataset. Sometimes, we used a skin tracing method for patient registration. All procedures, except DTI image en-coloration, were equal to traditional navigation system. Pre-built fiber tracking source data were transferred to the navigation station and the fused images were visualized after an automatic image fusion process. After registration, repeated landmark checks were performed during surgery to ensure the accuracy of overall ongoing clinical application.
During the operation, the use of the functional neuronavigation was identical to that for traditional navigation. The position of the navigation pointer tip in the surgical field was displayed on the workstation's monitor with the corresponding location in the image. Right after the fiber tracking procedures, all fiber bundles were displayed in the same color, after which we could color each fiber in different colors. During resection of the tumor, we frequently checked the location of target fiber tracts. To compensate for inaccuracy caused by brain shift, we tried to minimize brain retraction and to delay the timing of cerebrospinal fluid drainage or ventricle opening. We frequently checked anatomical landmarks, such as bony structures and brain cortex, to limit inaccuracy, as well. Under guidance of functional neuro-navigation and other modalities, we tried to preserve essential white matter tracts to prevent postoperative neurologic deficits and so that the tumors could be safely resected as radically as possible.
After operation, we routinely checked MRI scans including DTI within 48 hours to minimize postoperative artifacts. Extent of resection was classified by a neuro-radiologist as gross total resection (GTR) if no residual enhancement was noted on postoperative MRI scans, subtotal resection (STR) if residual enhancement was noted on postoperative MRI scans less than 10% imaging, and partial resection (PR) for residual enhancement more than 10%. In cases of non-enhancing tumor, fluid attenuated inversion recovery (FLAIR) abnormalities were used to determine the preoperative extent of tumor (similar to enhancing lesions). In these cases, the postoperative presence of residual FLAIR signals that corresponded to tumor on preoperative MR imaging was classified as GTR, STR, or PR in the same manner as for enhancing tumors.
All the patients were followed for at least 12 months. We checked the patency of each fiber tract on postoperative DTI images. "Transient worsening" was defined in patients who recovered to his or her preoperative neurologic state within 3 months after operation.
To evaluate the relationship between distance to the essential fiber tract from the lesion and postoperative neurologic outcomes, we classified the distance according to the "1 cm rule," lesions located beyond or within 1 cm.10,11 Neurological status was assessed in patients who underwent post-operative DTI, comparing pre and post-operative DTI.
Chi-square test was conducted to evaluate the relationship between the distance to essential fiber tracts and postoperative neurologic status. The correlation between neurologic status and DTI integrity was analyzed by Fisher's exact test. Statistical significance was defined as a p-value of less than 0.05. All statistical analyses were conducted using SPSS software package (version 17.0; SPSS Inc., Chicago, IL, USA).
Among 123 patients, GTR was achieved in 90 patients (73.2%), STR in 29 (23.6%), and PR in 4 (3.3%). The clinical characteristics of the study population are summarized in Table 1. A single white matter tract was inspected in 78 patients, and two or more white matter tracts were investigated in 45 patients. The numbers of interesting white matter tracts that were examined was 176 (corticospinal tract, 107; optic radiation, 31; arcuate fasciculus 38). The overall postoperative neurologic outcomes compared with preoperative function were as follows: 100 patients (81.3%) displayed no change or improvement in symptoms after operation, and in 16 patients (13.0%), neurologic status was transiently aggravated, but recovered completely within 3 months after operation. Unfortunately, 7 patients (5.7%) experienced postoperative permanent neurologic deterioration (additional or new neurologic aggravation).
The relationships between the distance between lesion and essential white matter tract and postoperative neurologic status are summarized in Table 2. Among 7 cases of permanent neurologic deterioration after operation, worsening of function was due to postoperative infarction in one patient and postoperative brain swelling in two patients. In the other 4 patients, neurologic deterioration was considered to be the result of direct damage to essential white matter tract. All permanent neurologic deficits occurred in the 1-0 cm group; none occurred in the ≥1 cm group. Nevertheless, there is no statistical significances between the two groups (p=0.074).
Seventy eight patients underwent post-operative DTI. Among them, 68 patients showed no neurologic change and intact white matter tract. Among the 7 patients who showed transient neurological deterioration, 6 patients showed preserved white matter tract, and 1 patient disruption of white matter tract. Among the 3 patients with permanent neurologic deterioration, 2 patients showed preserved white matter tract, and 1 patient showed disruption of white matter tract. There was no significant difference between transient and permanent deficit groups (p=0.300) (Table 3).
A 36-year-old man presented with seizures and headache for 2 months. Neurological examination showed no neurologic deficit. MRI scans revealed hyper intense lesion in the left insular lobe on T2-weighted images without enhancement (Fig. 1A and B). The DTI procedure was carried out to demonstrate a preoperative 3D reconstruction of mass and fibers (corticospinal tract and arcuate fasciculus for language), which showed that the mass was surrounded by arcuate fasciculus and corticospinal tract. Furthermore, some part of arcuate fasciculus fibers passed through the tumor (Fig. 1C). Considering preoperative neurologic status and the above imaging results, we decided to perform an awake craniotomy and tumor removal. During resection of the superior part of the mass adjacent to the arcuate fasciculus, naming aphasia developed, and so we stopped further resection (Fig. 1D). Postoperative MRI with DTI taken 2 days after operation verified small remnants of tumor and precise preservation of the arcuate fasciculus (Fig. 1E). The final histological diagnosis was anaplastic astrocytoma. After the operation, the patient exhibited no motor weakness or language problems. Three dimensional conformal radiation therapy was conducted postoperatively. The patient has shown no disease progression and no neurologic deficit during his 3 year follow-up evaluation.
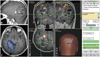
An 18-year-old female patient presented with seizures and headache for 1 month. Neurological examination showed no neurologic deficit and MRI scans revealed a heterogeneously enhanced cystic mass on T1-weighted imaging (Fig. 2A). The DTI procedure was carried out and showed that the optic radiation was located just below the mass (Fig. 2B). We performed total tumor resection and preservation of the optic radiation, which were verified by postoperative MRI and DTI taken 2 days after surgery (Fig. 2C). The final histological diagnosis was pilocytic astrocytoma. The patient has shown no neurologic deficit and no disease recurrence during her 3 year follow-up evaluation.
Since 1990, image-guided neurosurgery including neuronavigation has contributed to better outcomes for brain tumor surgery. However, anatomical neuronavigation only is not sufficient, especially in cases of surgery for lesions in or adjacent to eloquent areas. Therefore, many trials have been conducted to develop functional neuronavigation, which integrates functional images with anatomical images.1,7,11,12 Currently available functional imaging modalities include functional MRI, positron emission tomography, magnetoencephalography, and DTI for white matter tracts.
Diffusion tensor MRI is an imaging modality for detecting water molecules in tissue.6,13 In an isotropic circumstance, water molecules move randomly, but in white matter tracts, water molecules move along the axons of neurons anisotropically. Therefore, DTI can visualize specific white matter tracts of the central nervous system, and it has been widely used for research and clinical purposes in physiologic or pathologic conditions.14,15,16
Coenen, et al.17 reported a case study that integrated individual fiber tract mapping into a neuronavigational environment in two cases of glioblastomas. The study indicated that 3-D mapping of large fiber tracts and intraoperative use of neuronavigation mapping had the potential to increase the safety of neurosurgical procedures and to reduce surgical morbidity. After Coenen's report, many authors reported on the clinical application of DTI-merged neuronavigation. Nimsky, et al.1 reported their experience with 16 patients in whom the navigation images were merged for the pyramidal tract (n=14) or optic radiation (n=2). They concluded that fiber tract data can be reliably integrated into a standard neuronavigation system, allowing for intraoperative visualization of major white matter tracts, such as the pyramidal tract or optic radiation.
More recently, Wu, et al.2 reported a randomized prospective study of 238 cerebral glioma patients who were treated using DTI-based functional neuronavigation (FNN). They compared the surgical results of two groups that underwent DTI-merged neuronavigation or conventional neuronavigation. They reported that the FNN group showed significantly higher postoperative Karnofsky Performance Score (KPS) (86±20 versus 74±28, p<0.001) and longer median survival time among patients with high grade glioma (21.2 months versus 14.0 months).
As shown in previous studies, DTI-merged functional navigation seems to be more useful than conventional navigation in terms of visualization of white matter tracts of interest during surgery, and the preparation takes only about 30 minutes more than conventional navigation. It is also useful for preoperative surgical planning such as approach and resection control because 3D-constructed images can be derived from 2D axial images.
Some physicians do not like to use navigation due to errors caused by changes during operation, such as brain shift. However, in practicality, brain shift mainly occurs in only one direction, and the error might decrease when moving to deep portions because brain motion also decreases. When the brain sinks down due to brain shift during surgery, the real point should be located below than the point that the navigation indicates. Therefore, if operators could consider this brain shift effectively, the navigation could be still helpful for determining the surgical trajectory and defining the extent and direction of resection. Especially, navigation is useful for cortical localization, and it could be also useful for subcortical localization when it is combined with DTI.
The most ideal method to overcome brain shift is intraoperative image re-acquisition and re-tracking of DTI.12,18,19 However, considering the high cost of intraoperative MRI and the feasibility of its use, its practical utility is questionable. All things considered, functional neuronavigation is easy to use and more effective than conventional neuronavigation.
Our study comprised several metastatic tumor surgeries. Usually metastatic brain tumors have well demarcated margins; therefore, it is easy to think that there is no need for functional neuronavigation. However, we performed additional resection around such lesions to reduce recurrence, in which it was important to identify eloquent areas to prevent neurological deficits.20
Our study has some technical limitations. A critical limitation of this study is the lack of validation for DTI itself. The importance of validation of drawn white matter tracts is not in doubt. Leclercq, et al.21 compared calculated fiber tracts with subcortical language mapping, and reported that 81% (17 of 21) thereof correspond with subcortical mapping results, while four subcortical stimulations did not correspond with DTI data. They concluded that negative tractography does not rule out the persistence of a fiber tract, especially when invaded by a tumor. Also, the conventional limitations of neuro-navigation, such as brain shift errors, still remain.22 Also, DTI imaging itself is quite operator dependent (especially, postoperative DTI due to postoperative change), so there is a possibility of inter-operator bias or reproducibility errors.23
Considering these factors, one possible way to validate the results is to observe only the core of major white matter tracts using tractography and to compare them with anatomical knowledge, because trajectories and locations of the body of these tracts are fairly well known. Once the tracking data leaves the core and approaches the target gray matter regions, we do not have information to validate the results, especially for humans. A statistical approach introduced recently might prove to be a good technique to validate the results in terms of reproducibility.24,25
In conclusion, DTI could be easily integrated into navigational datasets and FNN could be helpful for maximal safe resection and preserving brain function. However, considering the limitations of this study and the lack of statistically significant results, this study should be regarded as preliminary. Further study should follow to overcome these limitations, and combination with additional methods, such as the awake mapping technique, could be essential to minimizing the limitations of FNN in certain cases.
Figures and Tables
Fig. 1
Thirty-six-year old male patient with an anaplastic astrocytoma at the left insula. The tumor mass shows poorly demarcated high signal intensity on T2-weighted MR images (A) and low signal intensity on T1-weighted MR images (B). During preoperative surgical planning (C), the arcuate fasciculus (red) was suspicious of partly passing through the tumor mass (purple) and the corticospinal tract (yellow) was surrounding the mass. When the superior part of the tumor was resected in an awake state (D), aphasia developed, and we stopped further resection of the tumor. Postoperative MRI with diffusion tensor image shows small remnants of tumor and a well preserved arcuate fasciculus (E).
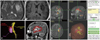
Fig. 2
Eighteen-year-old female patient with a pilocytic astrocytoma. The preoperative T1-weighted MR image shows a well enhanced cystic mass at the right occipital area (A). Navigation snap shot image (B) during operation reveals that the optic radiation is passing the medial margin of the tumor. Postoperative MRI with diffusion tensor image shows completely resected tumor and a well preserved optic radiation (C).
References
1. Nimsky C, Ganslandt O, Fahlbusch R. Implementation of fiber tract navigation. Neurosurgery. 2006; 58(4):Suppl 2. ONS-292–ONS-303.

2. Wu JS, Zhou LF, Tang WJ, Mao Y, Hu J, Song YY, et al. Clinical evaluation and follow-up outcome of diffusion tensor imaging-based functional neuronavigation: a prospective, controlled study in patients with gliomas involving pyramidal tracts. Neurosurgery. 2007; 61:935–948.
3. Qiu TM, Zhang Y, Wu JS, Tang WJ, Zhao Y, Pan ZG, et al. Virtual reality presurgical planning for cerebral gliomas adjacent to motor pathways in an integrated 3-D stereoscopic visualization of structural MRI and DTI tractography. Acta Neurochir (Wien). 2010; 152:1847–1857.

4. Golby AJ, Kindlmann G, Norton I, Yarmarkovich A, Pieper S, Kikinis R. Interactive diffusion tensor tractography visualization for neurosurgical planning. Neurosurgery. 2011; 68:496–505.

5. Moseley ME, Cohen Y, Kucharczyk J, Mintorovitch J, Asgari HS, Wendland MF, et al. Diffusion-weighted MR imaging of anisotropic water diffusion in cat central nervous system. Radiology. 1990; 176:439–445.

6. Stieltjes B, Kaufmann WE, van Zijl PC, Fredericksen K, Pearlson GD, Solaiyappan M, et al. Diffusion tensor imaging and axonal tracking in the human brainstem. Neuroimage. 2001; 14:723–735.

7. Nimsky C, Ganslandt O, Kober H, Moller M, Ulmer S, Tomandl B, et al. Integration of functional magnetic resonance imaging supported by magnetoencephalography in functional neuronavigation. Neurosurgery. 1999; 44:1249–1255.

8. Kober H, Nimsky C, Möller M, Hastreiter P, Fahlbusch R, Ganslandt O. Correlation of sensorimotor activation with functional magnetic resonance imaging and magnetoencephalography in presurgical functional imaging: a spatial analysis. Neuroimage. 2001; 14:1214–1228.

9. Ganslandt O, Fahlbusch R, Nimsky C, Kober H, Möller M, Steinmeier R, et al. Functional neuronavigation with magnetoencephalography: outcome in 50 patients with lesions around the motor cortex. J Neurosurg. 1999; 91:73–79.

10. Matz PG, Cobbs C, Berger MS. Intraoperative cortical mapping as a guide to the surgical resection of gliomas. J Neurooncol. 1999; 42:233–245.
11. Richardson RM, Berger MS. Neurophysiologic Mapping for Glioma Surgery: Preservation of Functional Areas. In : Lozano AM, Gildenberg PL, Tasker RR, editors. Textbook of Stereotactic and Functional Neurosurgery. Heidelberg: Springer-Verlag;2009. p. 325–334.
12. Nimsky C, Ganslandt O, Buchfelder M, Fahlbusch R. Intraoperative visualization for resection of gliomas: the role of functional neuronavigation and intraoperative 1.5 T MRI. Neurol Res. 2006; 28:482–487.

13. Westin CF, Maier SE, Mamata H, Nabavi A, Jolesz FA, Kikinis R. Processing and visualization for diffusion tensor MRI. Med Image Anal. 2002; 6:93–108.

14. Glasser MF, Rilling JK. DTI tractography of the human brain's language pathways. Cereb Cortex. 2008; 18:2471–2482.

15. Glenn OA, Ludeman NA, Berman JI, Wu YW, Lu Y, Bartha AI, et al. Diffusion tensor MR imaging tractography of the pyramidal tracts correlates with clinical motor function in children with congenital hemiparesis. AJNR Am J Neuroradiol. 2007; 28:1796–1802.

16. Lee MJ, Kim HD, Lee JS, Kim DS, Lee SK. Usefulness of diffusion tensor tractography in pediatric epilepsy surgery. Yonsei Med J. 2013; 54:21–27.

17. Coenen VA, Krings T, Mayfrank L, Polin RS, Reinges MH, Thron A, et al. Three-dimensional visualization of the pyramidal tract in a neuronavigation system during brain tumor surgery: first experiences and technical note. Neurosurgery. 2001; 49:86–92.

18. Nimsky C, Ganslandt O, Hastreiter P, Wang R, Benner T, Sorensen AG, et al. Preoperative and intraoperative diffusion tensor imaging-based fiber tracking in glioma surgery. Neurosurgery. 2005; 56:130–137.

19. Hirschberg H, Samset E, Hol PK, Tillung T, Lote K. Impact of intraoperative MRI on the surgical results for high-grade gliomas. Minim Invasive Neurosurg. 2005; 48:77–84.

20. Yoo H, Kim YZ, Nam BH, Shin SH, Yang HS, Lee JS, et al. Reduced local recurrence of a single brain metastasis through microscopic total resection. J Neurosurg. 2009; 110:730–736.

21. Leclercq D, Duffau H, Delmaire C, Capelle L, Gatignol P, Ducros M, et al. Comparison of diffusion tensor imaging tractography of language tracts and intraoperative subcortical stimulations. J Neurosurg. 2010; 112:503–511.

22. Nabavi A, Black PM, Gering DT, Westin CF, Mehta V, Pergolizzi RS Jr, et al. Serial intraoperative magnetic resonance imaging of brain shift. Neurosurgery. 2001; 48:787–797.

23. Lee SK, Kim DI, Kim J, Kim DJ, Kim HD, Kim DS, et al. Diffusion-tensor MR imaging and fiber tractography: a new method of describing aberrant fiber connections in developmental CNS anomalies. Radiographics. 2005; 25:53–65.





 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download