Abstract
Purpose
We sought to evaluate the clinical usefulness of decision making by a multidisciplinary heart team for identifying potential candidates for transcatheter aortic valve implantation (TAVI) in patients with symptomatic severe aortic stenosis.
Materials and Methods
The multidisciplinary team consisted of two interventional cardiologists, two cardiovascular surgeons, one cardiac imaging specialist, and two cardiac anesthesiologists.
Results
Out of 60 patients who were screened as potential TAVI candidates, 31 patients were initially recommended as appropriate for TAVI, and 20 of these 31 eventually underwent TAVI. Twenty-two patients underwent surgical aortic valve replacement (AVR), and 17 patients received only medical treatment. Patients who underwent TAVI and medical therapy were older than those who underwent surgical AVR (p<0.001). The logistic Euroscore was significantly highest in the TAVI group and lowest in the surgical AVR group (p=0.012). Most patients in the TAVI group (90%) and the surgical AVR group (91%) had severe cardiac symptoms, but only 47% in the medical therapy group had severe symptoms. The cumulative percentages of survival without re-hospitalization or all-cause death at 6 months for the surgical AVR, TAVI, and medical therapy groups were 84%, 75%, and 28%, respectively (p=0.007, by log-rank).
Conclusion
TAVI was recommended in half of the potential candidates following a multidisciplinary team approach and was eventually performed in one-third of these patients. One-third of the patients who were initially considered potential candidates received surgical AVR with favorable clinical outcomes.
Aortic stenosis disease (AS) is a common native valve disease found in up to 5% of the elderly population.1 Surgical aortic valve replacement (AVR) is the standard treatment for patients with symptomatic severe AS.2 However, despite the accepted results of conventional surgery, surgical risk is markedly increased in elderly patients with comorbidities. Additionally, several registries have demonstrated that about one-third of patients are considered too high-risk for conventional open heart surgery and remain untreated.3,4 Transcatheter aortic valve implantation (TAVI) was developed as an alternative to surgical AVR in this high-risk patient population. TAVI does not require cardiopulmonary bypass or sternotomy. Thus, identification of high-risk surgical patients is imperative for adequate selection of TAVI candidates. Although a number of methods for risk stratification of patients for cardiac surgery have been reported, they have limitations because their ability to predict actual events is reduced in high-risk and elderly patients.5,6 Moreover, no TAVI-specific scoring system for those patients has been developed.7,8 Therefore, a multidisciplinary heart team approach is currently recommended from the early stages of patient selection.9 The importance of a multidisciplinary team approach with both cardiovascular surgeons and cardiologists may be highlighted when performing TAVI with required surgical procedures or as back-up and particularly in a learning period of TAVI.8,10,11
The purpose of this study was to examine the impact of this multidisciplinary heart team approach on our clinical practice in relation to patient selection, treatment, and outcomes in patients with symptomatic severe AS.
In January 2011, we began screening patients as potential TAVI candidates. Between January 2011 and June 2012, 60 consecutive patients were prospectively screened as potential TAVI candidates because they were presumed to be at high operative risk (logistic Euroscore >20% or Society of Thoracic Surgeons score >10% or other conditions related to a high operative risk such as significant frailty). They underwent clinical assessment with transthoracic echocardiography. Severe AS was defined as an aortic valve area <0.8 cm2, a peak aortic jet velocity >4.0 m/s, or mean aortic valve pressure gradient ≥40 mm Hg as seen with transthoracic echocardiography. Of the 60 screened patients, one patient had mild cardiac symptoms according to the New York Heart Association (NYHA) functional class I. Thus, 59 patients with symptomatic severe AS underwent work-up for technical feasibility for TAVI. All patients underwent cardiac computed tomography and transesophageal echocardiography to define aortic annulus dimensions and to evaluate associated anatomical structures for performing TAVI. Moreover, aorta computed tomography was performed to determine the technical feasibility of the TAVI access site. This study was approved by the Institutional Review Board of our institute, and written informed consent was obtained from each patient.
After the systematic work-up, either TAVI or surgical AVR was recommended by our multidisciplinary heart team. This team consisted of two interventional cardiologists, two cardiothoracic surgeons, one cardiac imaging specialist, and two cardiac anesthesiologists. When conventional open heart surgery was considered to be too risky, TAVI was recommended to the patient. The Euroscore, comorbidities, frailty, and mobility were taken into account by the multidisciplinary heart team approach. During the multidisciplinary heart team approach, all patients separately and independently met and discussed their options with at least one interventional cardiologist, one cardiac imaging specialist, and two cardiovascular surgeons. Risks and benefits of the different treatment modalities in terms of survival, relief of symptoms, quality of life, and potential complications were described independently by each member of the multidisciplinary heart team. The decision making by the multidisciplinary heart team was determined in a conference meeting with attendance of all seven members and involved in-depth discussion and complete agreement of the treatment modalities among the seven members. This decision by the multidisciplinary heart team was reported to the patients, and final decisions were made by the patients.
All TAVI procedures were performed in a hybrid operating room with a specially equipped angiography system. The procedure was previously reported in detail.12,13,14 Briefly, the femoral artery was the preferred access site. Subclavian (n=2) or transaortic access (n=1) was considered when femoral access was not suitable for advancing the large vascular sheath. All patients had transvenous temporary cardiac pacing during the procedure. Balloon valvuloplasty with rapid ventricular pacing (150 to 200 beats/min) was performed prior to prosthetic valve deployment in 11 patients. Positioning and deployment of the prosthetic valve was performed under fluoroscopic guidance. All patients were implanted with a self-expandable prosthesis, the AccuTrak CoreValve System (Medtronic, Minneapolis, MN, USA) under general anesthesia. Valve sizes of 26 mm (n=10), 29 mm (n=9), and 31 mm (n=1) were used according to the annulus diameter. Immediately after deployment of the prosthetic valve, transesophageal echocardiography was performed to confirm good motion of the prosthetic valve and to identify any paravalvular leakage. Post-stent balloon dilation was performed in five patients to relieve paravalvular leakage.
Procedural burden (procedure time and length of intensive care unit stay) and safety outcomes (all-cause mortality, major stroke, major vascular complications, and acute kidney injury) during hospitalization were compared between the TAVI and surgical AVR groups. The definition of each outcome was in accordance with the Valve Academic Research Consortium guidelines.9 Moreover, clinical efficacy at 3 and 6 months was evaluated as the composite events of all-cause mortality and re-hospitalization due to severe AS or complications of TAVI or surgical AVR.
Continuous variables were compared using analysis of variance or Student's t-tests, and categorical variables and frequencies were compared using the chi-square test or Fisher's exact test. Survival curves were constructed using the Kaplan-Meier method and compared with the log-rank test according to the TAVI, surgical AVR, and medical therapy groups. All analyses were conducted using SPSS Statistics (version 18.0.0, IBM Corp., Armonk, NY, USA). A p-value <0.05 was considered statistically significant.
Of the 59 symptomatic (NYHA functional class ≥II) patients screened as TAVI candidates, the multidisciplinary heart team determined that 31 patients (53%) were appropriate candidates for TAVI, 26 patients (44%) for surgical AVR, and 2 patients (3%) for medical therapy (Fig. 1). The reasons for declining TAVI in 11 of 31 TAVI-recommended patients were disagreement of risk or complications of TAVI due to a less severe symptomatic status in five patients and refusing further intervention despite severe symptoms in six patients. The reasons for declining surgical AVR in four of the 26 surgical AVR-recommended patients were disagreement of risk or complications of surgery due to a less severe symptomatic status in three patients and refusing further intervention despite severe symptoms in one patient. Therefore, TAVI and surgical AVR were finally performed in 20 and 22 patients, respectively. Medical therapy without any aortic valve interventions was performed in 11 patients who refused TAVI, four patients who refused surgical AVR, and two patients who had moderate AS (after detailed assessment and confirmation with transesophageal echocardiography). Two patients with moderate AS were excluded for outcome analysis.
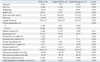
The baseline clinical characteristics are presented in Table 1. Patients receiving TAVI or medical therapy were older than those who underwent surgical AVR (p<0.001). The body mass index was significantly lower in patients receiving TAVI or medical therapy than in those receiving surgical AVR. The Society of Thoracic Surgeons risk score and the logistic Euroscore were significantly highest in the TAVI group and lowest in the surgical AVR group (p=0.047 and p=0.012, respectively). Most patients in the TAVI group and the surgical AVR group had severe cardiac symptoms (90% and 91% had NYHA class III or IV status). In contrast, only 47% in the medical therapy group had NYHA class III or IV status. Comorbidities were similar among the three groups except for more common peripheral artery disease in the TAVI group (p=0.026).
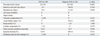
Procedural characteristics of TAVI and surgical AVR are shown in Table 2. In the TAVI group, the procedures were successfully performed in all patients. Four patients required a surgical approach for vascular access; two patients required transaortic access, one patient required subclavian access, and one patient required femoral cut-down. Vascular complications occurred in two patients; femoral artery occlusion after use of a closure device requiring vascular surgery in one patient and cardiac tamponade due to right ventricular rupture by rapid ventricular pacing requiring emergency open thoracotomy after a successful TAVI procedure in the other patient. In the surgical AVR group, the incidence of acute kidney injury was significantly higher than in the TAVI group (41% vs. 0%, p=0.001). Two out of nine patients who had acute kidney injury required dialysis. Moderate paravalvular leakage was observed in two patients (10%) in the TAVI group.
Three months after the procedures, one patient (5%) in the TAVI group, one patient (5%) in the surgical group, and four patients (27%) in the medical therapy group had died. The all-cause mortality at 3 months was not significantly different among the three groups (p=0.060). However, cardiac symptoms were significantly improved in both the TAVI group and the surgical AVR group (Table 3). Permanent pacemaker implantation was performed in one patient 3 months after TAVI. During a median follow-up period of 205 days, Kaplan-Meier analysis revealed that the cumulative survival rates at 6 months were 88%, 81%, and 51% for the surgical AVR, TAVI, and medical therapy groups, respectively (p=0.019, by log-rank) (Fig. 2A). Moreover, the rates of cumulative survival rate without re-hospitalization or all-cause death at 6 months were 84%, 75%, and 28% for the surgical AVR, TAVI, and medical therapy groups, respectively (p=0.007, by log-rank) (Fig. 2B).
The main findings of this prospective study for evaluation of a multidisciplinary heart team approach are that 1) half of the potential candidates for TAVI were recommended for TAVI, and about the other half of the potential candidates for TAVI were recommended for surgical AVR through this multidisciplinary approach; 2) surgical AVR, TAVI, and medical treatment were eventually performed in similar numbers of potential candidates for TAVI, following the patient's final decision and the multidisciplinary heart team's decisions; 3) although the surgical AVR group and the TAVI group showed favorable 6-month clinical outcomes, the medical treatment group showed worse 6-month clinical outcomes.
TAVI has emerged as an alternative treatment modality for high-risk or inoperable patients.12,15,16 Although a number of methods for risk stratification for cardiac surgery are commonly used to identify high-risk or inoperable patients, these stratification methods are limited in their ability to predict early operative outcomes in high-risk or elderly patients.5,6 Therefore, at least one surgeon, an interventionist, and a cardiac imaging specialist commonly review the same patient and make joint decisions to prevent inappropriate recommendation for procedures that will not clinically benefit those patients.9 A recent randomized study also introduced the concept of a multidisciplinary approach to select patients to undergo TAVI; two independent cardiovascular surgeons needed to agree that a patient was not eligible for conventional surgery and therefore could be included in that study.17 However, despite underscoring the clinical significance of patient selection through a multidisciplinary approach, little data exist regarding detailed decision-making processes by a multidisciplinary heart team for potential candidates for TAVI. One study reported favorable clinical outcomes following TAVI after a multidisciplinary meeting review of 386 TAVI-screened patients; TAVI was performed in 151 patients (39%), balloon valvuloplasty alone in 49 patients, surgical AVR in 48 patients (12%), and medical treatment in 104 patients (27%).18 Findings in our study, following a multidisciplinary heart team conference, TAVI and surgical AVR were initially recommended in 31 (53%) and 26 (44%) of 59 potential TAVI candidates, respectively. Compared to the TAVI and medical treatment groups, patients undergoing surgical AVR had a more favorable physical status (i.e., younger age, higher body mass index, and lower Society of Thoracic Surgeons score and logistic Euroscore). Consequently, patients who underwent surgical AVR showed comparable results as those who underwent TAVI. Therefore, careful patient selection through a multidisciplinary heart team approach led to satisfactory outcomes for patients with symptomatic severe AS not only in the TAVI group but also in the surgical AVR group. Moreover, the team approach enabled adequate selection of relatively lower-risk patients who are suitable candidates for surgical AVR with good results. This approach could lead to reconsideration of surgery in some selected patients who were initially considered high risk for surgery by other hospitals.
In the decision-making process for aortic valve intervention, patients must actively participate in the final treatment decision. Patients must be informed of the upfront risk of death, stroke, pacemaker placement, and major vascular complications. In our study, all patients were separately informed of selection of treatment modalities with at least one interventional cardiologist, one cardiac imaging specialist, and two cardiovascular surgeons during the multidisciplinary team approach. From this process, 11 patients (36%, 11/31) who were recommended for TAVI and four patients (15%, 4/26) who were recommended for surgical AVR refused further aortic valve interventions. Although this number may seem high, considering previous reports that surgical AVR was performed in only one-third to half of patients with severe AS in a general clinical practice, it is not as high as one can assume.4,19 One of the most common reasons for refusal was a less severe symptomatic status, which is similar to previous reports.19 Of note, however, these patients who did not undergo either TAVI or surgical AVR had the worst outcomes of the three groups. The important clinical decision may sometimes be made by the physicians alone without sufficient informed discussion, but an in-depth discussion between the multidisciplinary heart team and the patients before and after the procedures is crucial for further improvement of long-term clinical outcomes in patients with severe symptomatic AS. It was also interesting to note that there is such a high prevalence of refusal of intervention in the population even with severe symptoms, especially in those patients who were offered TAVI, with 19% (6/31) in the TAVI group and 4% (1/26) in the surgical AVR group. The introduction of new procedure, TAVI, without sufficient long term outcome might have influenced the confidence of patients. Also, the substantial costs of TAVI could be another explanation for high prevalence of refusal of TAVI.
This study has some limitations. The number of study patients was relatively small, and the follow-up duration was too short to reach concrete conclusions of the clinical significance of the multidisciplinary heart team approach. We did not perform additional objective tests to verify the severity of symptoms such as 6-minute walk test, which may have an additional prognostic value and be helpful for monitoring the patients in the medical therapy group.
In conclusion, a multidisciplinary heart team approach is essential for selecting ideal TAVI candidates from patients with symptomatic severe AS. This approach may help to select patients who have relatively favorable physical characteristics and can undergo surgical AVR, resulting in improved clinical outcomes.
Figures and Tables
 | Fig. 1Flow diagram of the multidisciplinary heart team approach. *Two patients offered medical therapy by multidisciplinary heart team because of moderate AS were excluded from outcome analysis. AVR, aortic valve replacement; CT, computed tomography; NYHA, New York Heart Association; TTE, trans-thoracic echocardiogram; TEE, trans-esophageal echocardiogram; TAVI, transcatheter aortic valve implantation. |
 | Fig. 2Kaplan-Meier survival curves for cumulative survival (A) and cumulative survival without rehospitalization or all-cause death (B). AVR, aortic valve replacement; TAVI, transcatheter aortic valve implantation. |
ACKNOWLEDGEMENTS
This study was supported by a grant from the Korea Healthcare Technology R&D Project, Ministry for Health, Welfare & Family Affairs, Republic of Korea (nos. A085012 and A102064), a grant from the Korea Health 21 R&D Project, Ministry of Health & Welfare, Republic of Korea (no. A085136), and the Cardiovascular Research Center, Seoul, Korea.
References
1. Nkomo VT, Gardin JM, Skelton TN, Gottdiener JS, Scott CG, Enriquez-Sarano M. Burden of valvular heart diseases: a population-based study. Lancet. 2006; 368:1005–1011.

2. Bonow RO, Carabello BA, Chatterjee K, de Leon AC Jr, Faxon DP, Freed MD, et al. 2008 focused update incorporated into the ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to revise the 1998 guidelines for the management of patients with valvular heart disease). Endorsed by the Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2008; 52:e1–e142.
3. Iung B, Baron G, Butchart EG, Delahaye F, Gohlke-Bärwolf C, Levang OW, et al. A prospective survey of patients with valvular heart disease in Europe: The Euro Heart Survey on Valvular Heart Disease. Eur Heart J. 2003; 24:1231–1243.

4. Freed BH, Sugeng L, Furlong K, Mor-Avi V, Raman J, Jeevanandam V, et al. Reasons for nonadherence to guidelines for aortic valve replacement in patients with severe aortic stenosis and potential solutions. Am J Cardiol. 2010; 105:1339–1342.

5. Osswald BR, Gegouskov V, Badowski-Zyla D, Tochtermann U, Thomas G, Hagl S, et al. Overestimation of aortic valve replacement risk by EuroSCORE: implications for percutaneous valve replacement. Eur Heart J. 2009; 30:74–80.

6. Leontyev S, Walther T, Borger MA, Lehmann S, Funkat AK, Rastan A, et al. Aortic valve replacement in octogenarians: utility of risk stratification with EuroSCORE. Ann Thorac Surg. 2009; 87:1440–1445.

7. Piazza N, Wenaweser P, van Gameren M, Pilgrim T, Tzikas A, Otten A, et al. Relationship between the logistic EuroSCORE and the Society of Thoracic Surgeons Predicted Risk of Mortality score in patients implanted with the CoreValve ReValving system--a Bern-Rotterdam Study. Am Heart J. 2010; 159:323–329.

8. Kempfert J, Rastan A, Holzhey D, Linke A, Schuler G, van Linden A, et al. Transapical aortic valve implantation: analysis of risk factors and learning experience in 299 patients. Circulation. 2011; 124:11 Suppl. S124–S129.
9. Kappetein AP, Head SJ, Généreux P, Piazza N, van Mieghem NM, Blackstone EH, et al. Updated standardized endpoint definitions for transcatheter aortic valve implantation: the Valve Academic Research Consortium-2 consensus document. J Am Coll Cardiol. 2012; 60:1438–1454.

10. Alli OO, Booker JD, Lennon RJ, Greason KL, Rihal CS, Holmes DR Jr. Transcatheter aortic valve implantation: assessing the learning curve. JACC Cardiovasc Interv. 2012; 5:72–79.
11. Gurvitch R, Tay EL, Wijesinghe N, Ye J, Nietlispach F, Wood DA, et al. Transcatheter aortic valve implantation: lessons from the learning curve of the first 270 high-risk patients. Catheter Cardiovasc Interv. 2011; 78:977–984.
12. Grube E, Schuler G, Buellesfeld L, Gerckens U, Linke A, Wenaweser P, et al. Percutaneous aortic valve replacement for severe aortic stenosis in high-risk patients using the second- and current third-generation self-expanding CoreValve prosthesis: device success and 30-day clinical outcome. J Am Coll Cardiol. 2007; 50:69–76.

13. Sinning JM, Ghanem A, Steinhäuser H, Adenauer V, Hammerstingl C, Nickenig G, et al. Renal function as predictor of mortality in patients after percutaneous transcatheter aortic valve implantation. JACC Cardiovasc Interv. 2010; 3:1141–1149.

14. Im E, Hong MK, Ko YG, Shin DH, Kim JS, Kim BK, et al. Comparison of early clinical outcomes following transcatheter aortic valve implantation versus surgical aortic valve replacement versus optimal medical therapy in patients older than 80 years with symptomatic severe aortic stenosis. Yonsei Med J. 2013; 54:596–602.

15. Cribier A, Eltchaninoff H, Tron C, Bauer F, Agatiello C, Sebagh L, et al. Early experience with percutaneous transcatheter implantation of heart valve prosthesis for the treatment of end-stage inoperable patients with calcific aortic stenosis. J Am Coll Cardiol. 2004; 43:698–703.

16. Webb JG, Pasupati S, Humphries K, Thompson C, Altwegg L, Moss R, et al. Percutaneous transarterial aortic valve replacement in selected high-risk patients with aortic stenosis. Circulation. 2007; 116:755–763.

17. Smith CR, Leon MB, Mack MJ, Miller DC, Moses JW, Svensson LG, et al. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med. 2011; 364:2187–2198.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download